Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4396
Revised: August 20, 2004
Accepted: August 22, 2004
Published online: July 28, 2005
AIM: Trace elements (TE) metabolism is altered in inflammatory bowel diseases. TE (zinc and copper) are constituents of antioxidant enzymes. Iron is involved in the pathogenesis of chronic inflammation. The aim was to evaluate zinc and copper status and the effects of iron manipulation in experimental colitis.
METHODS: Twenty-four male Sprague-Dawley rats were divided into four groups: standard diet, iron-deprived diet, iron-supplemented diet, and sham-treated controls. Macroscopic damage was scored. DNA adducts were measured in the colon. Liver and colonic concentration of TE were measured.
RESULTS: Macroscopic damage was reduced in iron-deprived groups and increased in iron-supplemented rats. Damage to the DNA was reduced in iron-deprived groups and increased in iron-supplemented groups. Liver and colonic iron concentrations were reduced in iron-deprived and increased in iron-supplemented rats. Liver zinc concentration was reduced after supplementation whereas colonic levels were similar in controls and treated rats. Liver copper concentration was reduced in all the colitic groups except in the iron-supplemented group whereas colonic concentration was increased in iron-deprived rats.
CONCLUSION: Iron deprivation diminishes the severity of DNBS colitis while supplementation worsens colitis. Zinc and copper status are modified by iron manipulation.
- Citation: Barollo M, D'Inc R, Scarpa M, Medici V, Cardin R, Bortolami M, Ruffolo C, Angriman I, Sturniolo G. Effects of iron manipulation on trace elements level in a model of colitis in rats. World J Gastroenterol 2005; 11(28): 4396-4399
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4396
Trace elements such as zinc and copper are essential for human health[1]. Zinc is required for cell membrane integrity, cell proliferation, and immune function. Several zinc-dependent antioxidant enzyme such as superoxide dismutase and metallothionein can neutralize free radicals production. Copper is necessary for the function of many enzymes involved in cell respiration and in cellular iron metabolism. Copper and zinc are both components of antioxidant enzymes such as superoxide dismutase. On the other hand, copper excess increases free radical levels thus enhancing the biological damage free radicals mediated[2].
In inflammatory conditions large amounts of reactive oxygen species are produced and this contributes with different mechanisms to damage tissue proteins, DNA chains and lipids[3]. Iron is a major peroxidative agent and animal studies demonstrated increased oxidative stress and intestinal inflammation after iron supplementation[4]. As previously reported by Katoet al[5] in Long Evans Cinammon rats, a model of copper liver toxicity, increased iron level is associated to copper excess and iron-deprived diet reduced mortality and fulminant hepatitis. Trace elements homeostasis is altered both in human and animal models of inflammatory bowel diseases with possible implication for disease activity and carcinogenesis[6-8].
We previously demonstrated that dietary iron deprivation is effective in reducing DNA damage and improves the outcome of colitis. The aim of this study was to evaluate the effects of iron supplementation compared to deprivation on disease activity, on trace elements status and on colonic DNA oxidative damage in a model of experimental colitis.
Twenty-four male Sprague-Dawley rats weighing 200 g were divided into four groups; one group was fed with standard diet containing 200 mg/kg of iron and given drinking water ad libitum. The second group was fed with an iron-controlled diet (50 mg/kg) and allowed to drink iron-free water for 5 wk and the third with an iron-supplemented diet (1 700 mg/kg) for 5 wk. The fourth group was fed with a standard diet (200 mg/kg of iron) and at the time of colitis induction was sham treated with saline.
Colitis was induced by the intrarectal instillation of 58 mg dinitro-benzene-sulfonic acid (DNBS) dissolved in 50% ethanol. The rats were anesthetized with ether and a silicone catheter was introduced intrarectally to 5 cm. Animals were kept in the Trendelemburg position for 10 min to avoid the rapid evacuation of the enema. On d 8, 1 wk after colitis induction, the animals were weighed and anesthetized with intraperitoneal chloral hydrate (400 mg/kg) after which the abdomen was opened with a midline incision and exsanguination was performed. The colon was removed, opened along the antimesenteric border, rinsed with iron-free water and weighed.
The damage was assessed by scoring the number and extension of ulcers, adhesions, and thickness of the colonic wall according to Morris et al[9].
Operators were unaware of the treatment of each group. Colonic tissue samples were obtained and processed for myeloperoxidase and 8-hydroxydeoxyguanosine (8-OHdG) determination and for measuring iron, zinc, and copper concentrations. Similarly liver samples were obtained for the determination of iron, zinc, and copper concentrations.
Trace elements concentrations were measured using atomic absorption spectrophotometry. Intestinal and liver tissues, obtained from rats, were dried at 42 °C for 24 h. The dried samples were weighed on an analytical balance, transferred into element-free tubes and then dissolved using 4.5 mL of 300 mL/L nitric acid solution. The tubes were incubated at 42 °C for 24 h. Iron, copper, and zinc standard solutions (0.05, 0.10, 0.20, 0.50, and 1 mg/mL) were prepared by dilution of concentrated stock solution (Titrisol, Merck Darmstadt, Germany) in deionized water. A Perkin Elmer 3100 atomic absorption spectrophotometer operated with an acetylene air mixture. A lean blue (oxidizing) flame was used with a cathode lamp current of 15 mA, a monochromator wavelength of 248.3 nm, and a slit width of 0.2 nm for Fe; lamp current of 25 mA, a wavelength of 213.9, and a slit width of 0.7 nm for Zn; lamp current of 15 mA, a wavelength of 324.8 nm, and a slit width of 0.7 mm for Cu.
Samples were aspirated directly and the concentration of the element of interest was determined from appropriate standard curves. Standard controls (Bovine liver, Trimital, Magenta, Milan, Italy) were prepared using the same extraction procedure used for sample preparation.
Results were expressed taking into account the dry weight and the dilution factor of the samples.
MPO activity was assessed following previously described methods[10]. Briefly colonic tissue samples were minced in 1 mL of 50 mmol/L potassium phosphate buffer (pH 6.0) containing 14 mmol/L hexadecyltrimethylammonium bromide (Fluka), homogenized and sonicated. The lysates have tol be frozen and thawed thrice, then centrifuged for 2 min in cold at 15 000 g. Aliquots of the supernatants were mixed with potassium phosphate buffer containing o-dianisidine-HCl (Sigma-Aldrich, St. Louis, MO, USA) and 0.0005% H2O2. MPO activity was expressed as units/g of wet tissue. The enzyme unit was defined as the conversion of 1 moL of H2O2 per minute at 25 °C.
Oxidative DNA damage was assessed following previously described methods[11]. Briefly colonic biopsy specimens were thawed, homogenized in a separation buffer and approximately 20 mg of purified DNA per sample was injected in the HPLC (Shimadzu, Kyoto, Japan). The 8-OHdG was detected using an electrochemical detector (ESA Coulochem II 5200A, Bedford, MA, USA). The levels of 8-OHdG were expressed as the number of 8-OHdG adducts per 105 dG bases. The coefficient of variation was <10%; 100 mg of DNA were required for the determination.
Data are expressed as mean +/- standard error. Statistical data were analyzed with Mann-Whitney U test for comparison of the four groups and Spearman's rank correlation test to evidence any relation between the evaluated parameters. P values less than 0.05 were considered significant.
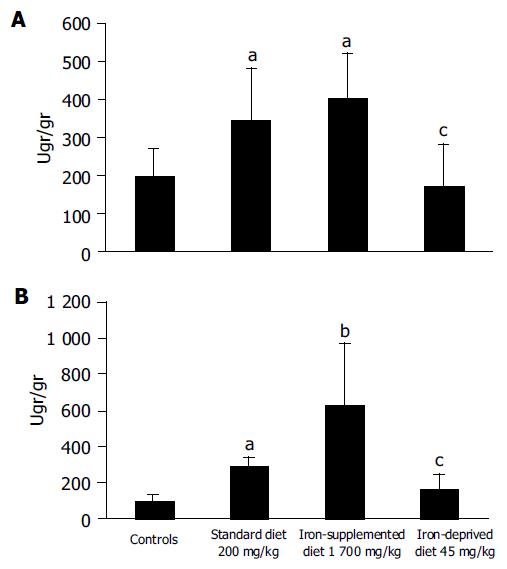
Before colitis was induced body weight was similar in all groups of animals. The colon weight, a rough measure of edema and inflammation, was significantly increased in colitic animals with respect to controls (P<0.05), while iron-deprived rats had colonic weights similar to controls. The macroscopic damage score was significantly lower in the group receiving iron-deprived diet than in the colitis groups. MPO activity was significantly increased in iron-supplemented rats. Iron deprivation was associated with significantly higher MPO levels than controls.
Clinical and biochemical aspects of colitis in all rat groups are summarized in Table 1.
Dietary iron deprivation significantly decreased hepatic and colonic iron concentrations. Iron supplementation increases the iron concentration in liver and colon compared to healthy controls. Iron concentration was increased in the liver and colon of rats with colitis (Fe, 200 mg/kg) whereas supplementation did not affect hepatic iron concentration (Figure 1). There was a significant correlation between hepatic and colonic iron concentrations (R = 0.567, P<0.002).
Inflammation significantly increased hepatic zinc concentration but neither iron deprivation or supplementation modified zinc concentration in the liver. Although no significant change was revealed in any of the treatments the more the colon was damaged the lower was the colonic zinc concentration (R = -0.460, P = 0.02).
Hepatic copper concentration was reduced in all colitic groups with respect to controls except in the iron-supplemented group. On the other hand, copper colonic concentration was increased in the iron-deprived diet group irrespective of treatment and inflammatory status (Table 2). Hepatic copper concentration correlated with colonic copper (R = 0.39, P<0.04).
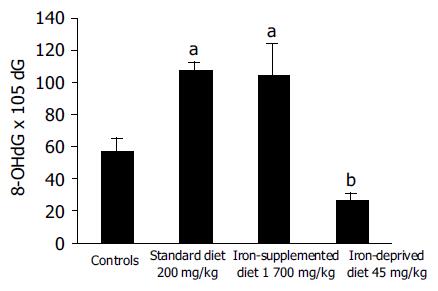
Colonic DNA adducts were significantly reduced in rats fed with an iron-deprived diet for 5 wk (Figure 2). Colonic DNA adducts significantly correlated with iron colonic concentration (R = 0.44, P<0.02).
Iron has a major role in chronic inflammatory diseases. Lin et al[12] demonstrated in vitro that iron chelation effectively blocks NF-kappa B activation and upregulates TNF-a and IL-6 genes in a model of cholestatic liver injury, suggesting a basic role for iron in the activation of the inflammatory process. In patients with Crohn's disease and anemia, treatment with oral ferrous fumarate decreased cysteine and glutathione peroxidase with consequent altered plasma antioxidant status[13]. Moreover, oxidative stress is increased in vitro cell lines from patients with ulcerative colitis treated with iron[14].
Recent evidence showed that iron dietary deprivation is a reasonable approach to many diseases with a free radical component[15]. It is known that Deferoxamine, an iron chelating agent, effectively reduces mucosal oxidant activity by decreasing the luminol-amplified chemiluminescence in vitro by 44% in active ulcerative colitis biopsies[16]. This effect was attributed more to iron chelation than to a direct antioxidant activity.
We observed that iron deprivation was associated with less macroscopic colonic mucosal damage while iron supplementation worsened colitis. Iron and inflammation seem to have a synergic action since MPO levels were greatly increased in the iron-supplemented group. Our observations suggest that iron manipulation may modulate inflammatory damage.
The results of this study confirm that dietary iron deprivation reduces inflammation and oxidative DNA damage in the rat model of DNBS-induced colitis while iron supplementation worsens colitis as we previously reported (in press)[17]. These results further reinforce our previous findings on the role of iron deprivation in DNBS colitis. Carrier et al[18] recently reported that oral iron supplementation may aggravate inflammation and oxidative stress in dextran sulfate sodium-induced colitis. Oxidative damage, expressed by DNA adducts level, was decreased in iron-deprived rats. According to Seril et al[19] reactive oxygen species, produced in abundance in the presence of iron during inflammation, can directly mediate DNA damage thus leading to alterations, which cause loss of suppressor genes and gain of oncogenes function.
Trace elements are altered during inflammation and their status is critical for normal cell and enzymes function. Many enzymes, involved in DNA repair mechanisms, are zinc-dependent thus trace elements alteration could contribute to DNA damage[20]. Several human and animal studies have demonstrated altered trace elements status during inflammation. Al Awadi et al[8] reported a significant reduction of colonic zinc level in experimental colitis while copper and manganese remained unaltered. Zinc and copper serum levels were altered in well-nourished patients with ulcerative colitis and correlated with hematological parameters of disease activity suggesting their role in inflammation[21]. We previously demonstrated that zinc supplementation regulates tight junction permeability in experimental colitis with possible implication on mucosal healing[22]. Several studies have pointed out that zinc, copper, and iron may affect the progression of colonic tumors in experimental model of preneoplastic lesions[23]. Moreover, Ames has recently reported that zinc and other micronutrient deficiencies mimic the effect of radiation on DNA chain with strong implication for carcinogenesis[24].
Hepatic zinc concentration is significantly reduced during iron dietary deprivation. Colonic zinc concentration is similar to controls in all treated groups and it is independent from iron metabolism. The inverse correlation with macroscopic score and colonic weight may suggest the relevant effect of zinc on mucosa healing. In fact as recently reported by Kruidenier superoxide dismutase Zn/Cu dependent is decreased in colonic mucosa of IBD patients with active inflammation[25].
Colonic copper is increased during iron dietary deprivation. Iron supplementation does not seem to affect copper absorption as recently demonstrated in ileostomy subjects[26]. Colonic copper alterations seem therefore a consequence of local inflammation. On the other hand our data showed that inflammation decreased copper concentration in the liver except in the presence of iron supplementation. This is in agreement with the results reported in Long Evans Cinammon rats, in which an iron deprived diet reduced mortality and fulminant hepatitis[5].
In conclusion, we pointed out that iron manipulation affects the severity of experimental colitis. Iron manipulation results in changes of zinc and copper status which may, after a chemical insult, alter the natural course of intestinal inflammation and may have important implications for the development of antioxidant treatment of IBD patients.
Science Editor Zhu LH Language Editor Elsevier HK
| 1. | Bogden JD, Klevay LM. Clinical nutrition of the essential trace elements and minerals. The guide for health professionals. 2000;Humana Press Inc. [DOI] [Full Text] |
| 2. | Olivares M, Uauy R. Copper as an essential nutrient. Am J Clin Nutr 1996; 63: 791S-796S 9 Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;98:795-803. |
| 3. | Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 311] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Reifen R, Matas Z, Zeidel L, Berkovitch Z, Bujanover Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig Dis Sci. 2000;45:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Kato J, Kobune M, Kohgo Y, Sugawara N, Hisai H, Nakamura T, Sakamaki S, Sawada N, Niitsu Y. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long-Evans Cinnamon rats. J Clin Invest. 1996;98:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Ringstad J, Kildebo S, Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1993;28:605-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Brignola C, Belloli C, De Simone G, Evangelisti A, Parente R, Mancini R, Iannone P, Mocheggiani E, Fabris N, Morini MC. Zinc supplementation restores plasma concentrations of zinc and thymulin in patients with Crohn's disease. Aliment Pharmacol Ther. 1993;7:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Al-Awadi FM, Khan I, Dashti HM, Srikumar TS. Colitis-induced changes in the level of trace elements in rat colon and other tissues. Ann Nutr Metab. 1998;42:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 10. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 11. | Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN. DNA oxidation matters: the HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proc Natl Acad Sci USA. 1998;95:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 482] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Lin M, Rippe RA, Niemelä O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol. 1997;272:G1355-G1364. [PubMed] |
| 13. | Erichsen K, Hausken T, Ulvik RJ, Svardal A, Berstad A, Berge RK. Ferrous fumarate deteriorated plasma antioxidant status in patients with Crohn disease. Scand J Gastroenterol. 2003;38:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Millar AD, Rampton DS, Blake DR. Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Aliment Pharmacol Ther. 2000;14:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Polla BS. Therapy by taking away: the case of iron. Biochem Pharmacol. 1999;57:1345-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Carotenuto P, Pontesilli O, Cambier JC, Hayward AR. Desferoxamine blocks IL 2 receptor expression on human T lymphocytes. J Immunol. 1986;136:2342-2347. [PubMed] |
| 17. | Barollo M, D'Incà R, Scarpa M, Medici V, Cardin R, Fries W, Angriman I, Sturniolo GC. Effects of iron deprivation or chelation on DNA damage in experimental colitis. Int J Colorectal Dis. 2004;19:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Carrier J, Aghdassi E, Platt I, Cullen J, Allard JP. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 353] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Leon O, Roth M. Zinc fingers: DNA binding and protein-protein interactions. Biol Res. 2000;33:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Dalekos GN, Ringstad J, Savaidis I, Seferiadis KI, Tsianos EV. Zinc, copper and immunological markers in the circulation of well nourished patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 1998;10:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D'inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Davis CD, Feng Y. Dietary copper, manganese and iron affect the formation of aberrant crypts in colon of rats administered 3,2'-dimethyl-4-aminobiphenyl. J Nutr. 1999;129:1060-1067. [PubMed] |
| 24. | Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 360] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Kruidenier L, Kuiper I, van Duijn W, Marklund SL, van Hogezand RA, Lamers CB, Verspaget HW. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J Pathol. 2003;201:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Troost FJ, Brummer RJ, Dainty JR, Hoogewerff JA, Bull VJ, Saris WH. Iron supplements inhibit zinc but not copper absorption in vivo in ileostomy subjects. Am J Clin Nutr. 2003;78:1018-1023. [PubMed] |