Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4321
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: July 28, 2005
AIM: To describe a new classification method of right hepatectomy according to the different special positions of tumors.
METHODS: According to positions, 91 patients with malignant hepatic tumor in the right liver lobe were divided into six groups: tumors in the right posterior lobe and (or) the right caudate lobe compressing the right portal hilum (n = 14, 15.4%), tumors in the right liver lobe compressing the inferior vena cava and (or) hepatic veins (n = 11, 12.9%), tumors infiltrating diaphragmatic muscle (n = 7, 7.7%), tumors in the hepatorenal recess (infiltrating the right fatty renal capsule, transverse colon and right adrenal gland, n = 8, 8.8%), tumors deeply located near the vertebral body (n = 3, 3.3%), tumors at other sites in the right liver lobe (the control group, n = 48, 52.75%). The values of intraoperative blood loss (IBL), tumor’s maxim cross-section area (TMCSA), and time of hepatic hilum clamping (THHC) and incidence of postoperative complications were compared between five groups of tumor and control group, respectively.
RESULTS: The THHC in groups 1-4 was significantly longer than that in the control group, the IBL in groups 1-4 was significantly higher than that in the control group, the TMCSA in groups 2-4 was significantly larger than that in the control group, and the ratio of IBL/TMCSA in group 1 was significantly higher than that in the control group. There was no significant difference in the indexes between group 5 and the control group.
CONCLUSION: The site of tumor is the key factor that determines IBL.
- Citation: Fan N, Yang GS, Lu JH, Yang N. Classification of right hepatectomy for special localized malignant tumor in right liver lobe. World J Gastroenterol 2005; 11(28): 4321-4325
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4321
Clinically, 60% of malignant tumors occur in the right liver which has a great physical volume and complicated anatomic structure, with a large number of vessels inside and important organs around, such as diaphragmatic muscle, right kidney, right adrenal gland, colon, and inferior vena cava. Therefore, tumors in the right liver lobe with different sizes and diversified positions may induce a widely different series of problems in the process of resection of these tumors. Generally, the easily removed tumor has a diameter less than 5 cm and is located on the surface of liver without infiltrating the surrounding important organs, or does not compress the main blood vessels in the liver. The features of tumors which are different to resect are as follows. (1) The diameter of tumor is larger than 5 cm and the tumor is close to or even infiltrates the major vessels of liver; (2) Although the diameter of tumor is less than 5 cm, it protrudes from the liver surface infiltrating surrounding organs. The tumor is deeply located and hard to be exposed during surgery. The tumor which meets one or more of the above standards is defined as the malignant tumor within the right liver with special position (MTRLSP). For the much more intraoperative blood loss (IBL), the longer the time of hepatic hilum clamping (THHC), the more the postoperative complications. MTRLSP has been a difficulty in right hepatectomy. Little information is available on the classification of right hepatectomy for MTRLSP. This paper presents a prospective observation on the surgical features of 91 cases of right hepatectomies, and the classification according to the standards mentioned above.
Ninety-one patients with malignant tumor within the right liver underwent right hepatectomy from 2002-05 to 2003-09 at Eastern Hepatobiliary Surgery Hospital of Second Military Medical University. There were 73 male and 18 female patients, ranging 25-78 years of age, including 87 cases of primary liver cancer, 2 cases of colon metastatic cancer, 1 case of rectum metastatic cancer, and 1 case of liver lymphoma. There were four cases of tumor thrombosis in portal vein and two cases in the bile duct. No death occurred in hospital.
Forty-three cases of MTRLSPs were divided into five groups in light of tumor positions, and 48 cases of tumors at other sites within right liver lobe in the corresponding period served as the control (Table 1).
| Tumor position | Cases (%) | Total (%) |
| MTR | ||
| LSP Group 1 Tumors within right | 14/91 (15.4) | |
| posterior lobe and (or) | ||
| right caudate lobe | ||
| compressing right | ||
| portal hilum | ||
| Group 2 Massive tumors | 11/91 (12.09) | 43/91 (47.25) |
| within right liver lobe | ||
| compressing the inferior | ||
| vena cava and (or) | ||
| hepatic veins | ||
| Hepato-renal infiltrating | 7/91 (7.7) | |
| transverse colon | ||
| Recess infiltrating right | 4/91 (4.4) | |
| adrenal gland | ||
| Group 3 Tumors infiltrating | 3/91 (3.3) | |
| diaphragmatic muscle | ||
| Group 4 Tumors in the | 1/91 (1.1) | |
| infiltrating right | ||
| fatty renal capsule | ||
| Group 5 Tumors deeply located | 3/91 (3.3) | |
| near vertebral body | ||
| control | ||
| Group 6 Tumors in other places | 48/91 (52.75) |
The Pringle’s maneuver was routinely adopted in tumor resection to control bleeding. The THHC was recorded during surgery, and the IBL and the TMCSA were measured after surgery. The postoperative complication of each patient was also recorded. The IBL, THHC (used for evaluating tumor size), TMCSA and postoperative complications in each group were compared to those in the control group respectively.
The data were expressed as mean±SE. SPSS for Windows, version 11.0 was used with Dunnett-t or Wilcoxon signed rank test.
The values of THHC in each group are shown in Table 2. The time in groups 1-4 was much longer than that in group 6 (P<0.05), but not in group 5 (P>0.05). For F- test = 0.078>0.05, bilateral Dunnett-t was used.
The values of IBL in each group are shown in Table 3. The value of IBL in groups 1-4 was much greater than that in group 6 (P<0.05), but not in group 5 (P>0.05). For F-test = 0.007<0.05, bilateral Wilcoxon signed rank test was used.
The values of TMCSA in each group are shown in Table 4. The value of TMCSA in groups 2-4 was much larger than that in group 6 (P<0.05), but not in groups 1 and 5 (P>0.05). For F-test = 0.000<0.05, bilateral Wilcoxon signed rank test was used.
The ratios of IBL/TMCSA in each group are shown in Table 5. The value of IBL in groups 2-5 was not significantly higher than that in group 6 (P>0.05), but not in group 1 (P<0.05). For F-test = 0.114>0.05, bilateral Dunnett-t was used.
| Group | 1 | 2 | 3 | 4 | 5 | 6 |
| IBL/TMCSA (mL/cm2) | 38.14±9.91 | 14.81±2.79 | 22.85±6.76 | 17.13±2.61 | 34.33±20.98 | 13.42±2.24 |
| P values | 0.003a | 1.000 | 0.788 | 0.994 | 0.387 |
The rate of blood transfusion during surgery and the incidence of postoperative complications in each group are shown in Table 6, the above two values were all zero in groups 5 and 6. The main postoperative complication in groups 1, 2, and 4 was right pleural effusion. The main postoperative complications in group 3 were right pleural effusion and rupture of diaphragm.
| Group | 1 | 2 | 3 | 4 | 5 | 6 |
| Blood transfusion | 35.71 | 45.45 | 42.86 | 37.5 | 0 | 0 |
| Incidence of postoperative complications | 14.29 | 36.36 | 71.43 | 37.5 | 0 | 0 |
According to the definition of MTRLSP, the proportion of MTRLSP is 47.25% (Table 1), which shows that nearly half of right hepatectomies for malignant tumors are the high-risk resections for MTRLSPs. The IBL, THHC, and postoperative complications are the three risk factors for the resection for MTRLSP; tumor size and site determine these factors. In this study, taking the influence of tumor size as the index of IBL/TMCSA, we divided the MTRLSPs into five groups according to their positions to analyze and identify the main reason for high-risk resections of tumors in each group.
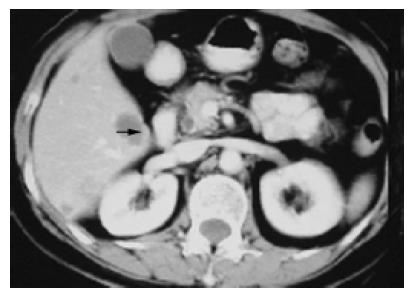
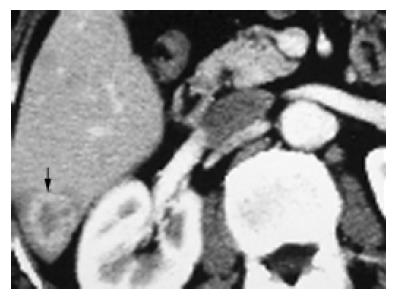
Tumors in the group 1 (n = 14, 15.4%) within the right posterior lobe and (or) right caudate lobe usually compressed or even infiltrated the right posterior portal vein (RPPV, Figure 1), occasionally with the tumor thrombus in vessels. Tumor thrombi almost all occurred in this (in portal branch, n = 3, 3/4 = 75%; in bile duct, n = 2, 2/2 = 100%). The tumor was usually located between the first and third hepatic hilum with some main blood vessels around, such as the RPPV, inferior vena cava (IVC). It was difficult to control bleeding in an extremely dangerous state once these main vessels rupture when the tumor was exposed, and to injure the first hepatic hilum if blind suture was used to stop bleeding. Therefore, the IBL (642.85±132.89 mL) was much greater than that in the control group (257.40±29.44 mL, P = 0.033), and the THHC (31.00±3.53 min) was also significantly longer than that in the control group (19.148± 1.27, P = 0.001), though there was no statistical difference (P = 0.683) in TMCSA between this group (20.61±4.67 cm2) and control group (22.77±3.67 cm2). The main reason for high-risk resection of tumors of this group is the site but not the size of tumors, which is further proved by the fact that the index of IBL/TMCSA (38.14±9.91) was significantly higher than that in the control group (13.42±2.24, P = 0.003).
For example, an operation of 1 patient with the tumor in the right caudate going down to left caudate ran into trouble. After the tumor was ablated from the wall of the compressed IVC and RPPV, it was impossible to suture the wound because the nude right portal branch was just on the surface of the incision wedge. A drainage tube was placed instead of suture of the wound. A bile leakage was found 3 d after surgery which was closed after drainage.
Generally, to handle the tumor at this site, we should remove it as soon as possible, and suture the liver wound by any possible way, and observe the characteristics of drainage carefully in case the severe bile leakage occurs after surgery[1].
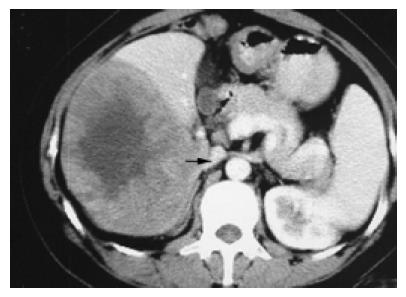
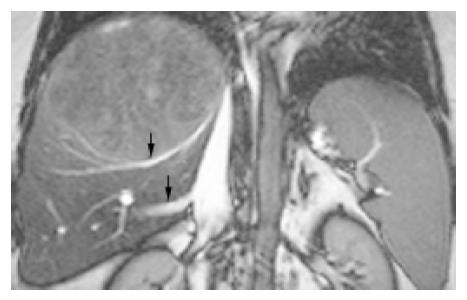
Tumors (n = 11, 12.9%) in group 2 compressing part or completely at the inferior vena cava (n = 6, Figure 2) and (or) hepatic veins (n = 4, Figure 3) had the largest size in groups 1-6, with TMCSA being 84.95±11.76 cm2, larger than that in the control group 22.77±3.67 cm2, P = 0.003. They were so large that they almost occupy the whole right liver lobe.
The IBL (1 054.54±200.16 mL) and THHC (36.72± 2.41 min) were much greater and longer than those in the control group (IBL: 257.40±29.44 mL, P = 0.005; THHC: 19.148±1.27 min, P = 0.000), suggesting a higher risk for surgery in this group. But there was no statistical difference in the index of IBL/TMCSA (14.81±2.79) between this group and control group (13.42±2.24,P = 1.000), suggesting that IBL is not much greater than that in the control group when the influence of tumor size is excluded. Apparently, the biggest tumor size is the main reason for the top 36 min THHC and 1 054 mL IBL in this group.
Some troubles caused by the compression of the IVC from tumors are as follows. When the tumor is removed from normal liver, severe bleeding may occur due to lacerated breakage on the IVC wall or crevasse of the hepatic short vein (HSV) with ligation falling off. After the tumor is removed, the remnant liver with full-length IVC uncovered on a wide surface of the incision wedge may be hard to be sutured for the fear of IVC obstruction due to compression from suture of wound. The obstruction can induce liver swelling and severe cirrhosis. The treatment strategies may be summarized as follows. (1) If the image of a tumor shows preoperatively that the tumor compresses or infiltrates the IVC, the blocking string should be preplaced around the superior and inferior IVC to the effect that severe bleeding can be easily controlled. The breakage is better sutured with 3-0 prolene, but not clamped. In addition, the HSV and the right adrenal gland vein should be abscised and ligated carefully; (2) To avoid compression of the IVC, an incision wedge should be shaped ‘arc’ so that both sides can be sutured with the IVC untouched at the bottom of the wound, and the remnant liver can be suspended to the abdomen wall. If the above measures do not work, the sutures of liver wound should be taken out, and then after careful hemostasis, the epiploon can be sutured to the incision wedge. Recently, some studies reported[2,3] that fibrin sealant can be ejected to the surface of liver wound to hemostasis, and results are quick and good.
The other sort of troubles comes from compression and infiltration of right hepatic veins (RHV) by a tumor under which there is usually a part of normal liver (Figure 3). Whether this part of normal liver should be excised with the tumor depends on the following two situations: (1) If a patient has severe liver cirrhosis and small left liver lobe, the normal liver in right posterior should be retained, and so does the RHV or dilated HSV to ensure blood flowing back[4]; (2) If a patient has mild liver cirrhosis and thick left liver lobe, the remnant liver and RHV can be excised with the tumor.
Tumors in group 3 (n = 7, 7.7%) had the highest value of IBL among all groups (1 085.71±310.47 mL vs the control 257.40±29.44 mL, P = 0.046). One reason is that tumors usually grow out of liver surface and adhere to diaphragm with abundant bypass blood vessels. The other reason is the large TMCSA (56.38±12.50 cm2vs the control 22.77±3.67 cm2, P = 0.028). The larger the tumor and the wider the interface between tumor and diaphragm, the more the bleeding during surgery. But there was no statistical difference in the index of IBL/TMCSA (22.85±6.76) between this group and control group (13.42±2.24, P = 0.788), suggesting that IBL is not much greater than that in the control group when the influence of tumor size is excluded. The higher IBL and bigger TMCSA induce longer THHC (31.42±4.56 min vs the control 19.148±1.27 min, P = 0.013) and higher incidence of postoperative complications (n = 5/7 = 71.43%, Table 6) such as right pleural effusion (n = 3) and diaphragm rupture (n = 2).
Tumors which infiltrate the diaphragm growing in an expansible fashion are usually too big to control during surgery, and should be excised integrally without the diaphragm being broken. But because of limited operational space for removal of surrounding ligaments around the liver, it is inevitable that tumors are occasionally oppressed. Once tumor raptures, a large quantity of low osmosis water should be used to wash the peritoneal cavity to prevent tumor planting. In addition, a part of the diaphragm approaching the liver is also flimsy and frangible[5] (Figure 4).
Thoracoabdominal approach for resection of this sort of tumors has been adopted by Xia et al[6]. They found that the IBL is less and better exposured to hemostasia than abdominal approach, without an increasing incidence of postoperative complications, but with increased trauma and more complicated operative procedure. Our study also found a higher volume of the IBL and a higher incidence of postoperative complications. Therefore, along with the development of surgical technique and perioperative intensive care, thoracoabdominal approach may be reasonable for the resection of this sort of tumors.
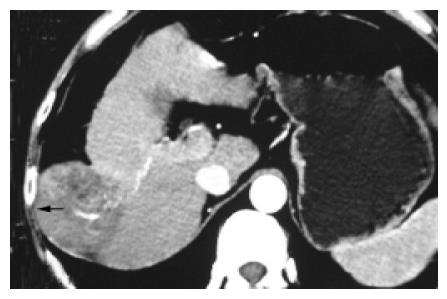
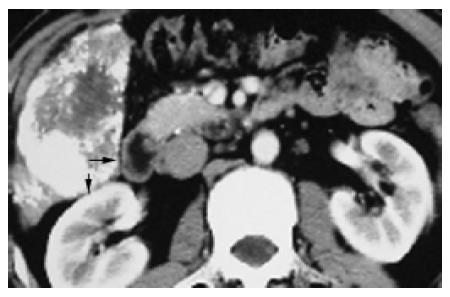
Tumors in group 4 (n = 8, 8.8%; infiltrating right fatty renal capsule n = 4, transverse colon n = 3, right adrenal gland, n = 1) protruding to hepatorenal recess were prone to infiltrate the right fatty renal capsule, transverse colon (Figure 5) or right adrenal gland and induce wide conglutination which caused the following difficulties. (1) Abundant bypass vessels between tumor and its surroundings were likely to bleed in the course of exploration and liberation. The IBL of this group (850.00±185.16 mL) was significantly greater than that in the control group (257.40±29.44 mL, P = 0.011); (2) Because of larger size (TMCSA 46.87±5.59 cm2vs the control 22.77±3.67 cm2, P = 0.012) and severe infiltration and conglutination with surrounding tissues, tumor became a mass which did not leave enough space for hands to move the liver lobe for liberating right ligaments. But there was no statistical difference in the index of IBL/TMCSA (14.81±2.79) between this group and the control group (13.42±2.24, P = 1.000). The retrograde hepatectomy can be used to deal with this situation, and this method has been used for difficult hepatectomy for years[7-9].
Tumors in group 5 were located within the right posterior-inferior segment (Figure 6), and difficult to be exposed during surgery, and had to be padded with absorbent gauze on the surface of post-parietal peritoneum to lift liver and tumors up for a good exposure after liberation of ligaments. As long as there was good exposure, it was not very difficult to remove the tumor in this group.
In conclusion, this study isolated the MTRLSP from malignant tumors in the right liver and classified them into five sorts in terms of their position. The surgical features of each sort are described, which may improve the resection rate of malignant tumors within the right liver lobe and reduce the complications after surgery.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
Co-first-authors: Guang-Shun Yang and Ning Fan
| 1. | Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, Takemura S, Yamamoto T, Uenishi T, Kinoshita H. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Davidson BR, Burnett S, Javed MS, Seifalian A, Moore D, Doctor N. Experimental study of a novel fibrin sealant for achieving haemostasis following partial hepatectomy. Br J Surg. 2000;87:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Liu M, Lui WY. The use of fibrin adhesive for hemostasis after liver resection. Zhonghua YiXue ZaZhi (Taipei). 1993;51:19-22. [PubMed] |
| 4. | Smyrniotis V, Arkadopoulos N, Kehagias D, Kostopanagiotou G, Scondras C, Kotsis T, Tsantoulas D. Liver resection with repair of major hepatic veins. Am J Surg. 2002;183:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Luo YQ, Wang Y, Chen H, Wu MC. Influence of preoperative transcatheter arterial chemoembolization on liver resection in patients with respectable hepatocelluar carcinoma. Zhonghua Putong Waike Zazhi. 2002;17:149-151. |
| 6. | Xia F, Poon RT, Fan ST, Wong J. Thoracoabdominal approach for right-sided hepatic resection for hepatocellular carcinoma. J Am Coll Surg. 2003;196:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Lin TY, Sridharan M, Ho ST. Retrograde resection of hepatic lobe for extensive carcinoma of the liver. Med Chir Dig. 1977;6:87-88. [PubMed] |
| 8. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314-317; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Wu ZQ, Fan J, Zhou J, Qiu SJ, Ma CZ, Zhou XD, Tang ZY. Retrograde hepatectomy and research on its procedure. Zhonghua Gandan Waike Zazhi. 1999;5:301-303. |