Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4167
Revised: December 20, 2004
Accepted: December 23, 2004
Published online: July 21, 2005
AIM: To build up the research models of hepatic fibrosis in mice.
METHODS: Inbred wild-type FVB/N mice were either treated with alpha-naphthyl-isothiocyanate (ANIT), allyl alcohol (AA), carbon tetrachloride (CCl4), 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), and silica, or subjected to common bile duct ligation (CBDL) to induce hepatic injury. Liver biopsies were performed every 4 wk to evaluate hepatic fibrosis over a period of 6 mo. Cumulative cirrhosis and survival curves were constructed by life table method and compared with Wilcoxon test.
RESULTS: Under the dosages used, there was neither mortality nor cirrhosis in AA and silica-treated groups. DDC and ANIT caused cirrhosis within 4-12 and 12-24 wk, respectively. Both showed significantly faster cirrhosis induction at high dosages without significant alteration of survival. The duration for cirrhosis induction by CCl4 ranged from 4 to 20 wk, mainly dependent upon the dosage. However, the increase in CCl4 dosage significantly worsened survival. Intraperitoneal CCl4 administration resulted in better survival in comparison with gavage administration at high dosage, but not at medium and low dosages. After CBDL, all the mice developed liver cirrhosis within 4-8 wk and then died by the end of 16 wk.
CONCLUSION: CBDL and administrations of ANIT, CCl4, and DDC ensured liver cirrhosis. CBDL required the least amount of time in cirrhosis induction, but caused shortened lives of mice. It was followed by DDC and ANIT administration with favorable survival. As for CCl4, the speed of cirrhosis induction and the mouse survival depended upon the dosages and the administration route.
- Citation: Chang ML, Yeh CT, Chang PY, Chen JC. Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation. World J Gastroenterol 2005; 11(27): 4167-4172
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4167
Being the final outcome of chronic liver damage regardless of the underlying etiologies, hepatic fibrosis demands thorough study. The studies of hepatic fibrosis rely upon the availability of animal models. Although no experimental model exactly reproduces human liver fibrosis by etiologies, animal models serve to improve our understanding of pathogenetic mechanisms of liver fibrosis. In view of the cost-effectiveness in inducing hepatic injuries, hepatotoxin administration and common bile duct ligation (CBDL) were considered as useful methods[1].
In recent years, advances in molecular biology have helped to generate a transgenic mouse model of liver fibrosis[2]. This represents a new form of perturbation analysis whereby selective expression of novel or altered genes are used to perturb a complex system so that the information about the changes during development, functioning and malfunctioning can be deduced[3]. This approach potentially allows development of animal models with liver fibrosis that are inducible and genetically similar to that of man. A reliable and reproducible model of hepatic fibrosis in wild-type control mice will aid to unveil the pathologic mechanisms deduced from genetic and molecular approaches. However, informative data of mouse hepatic fibrosis generated in the laboratory remained lacking in the literature because rats had long been the most common animal model for studying hepatic fibrosis induced by various chemical or surgical hepatic injuries[1]. Since different animal models display distinct characteristics in the nature of pathogenesis of fibrosis or cirrhosis, the data acquired from rats could not apply to mice without bias[4-8]. This urged us to set up a mouse model of hepatic fibrosis using CBDL or the administration of various hepatotoxins including alpha-naphthyl-isothiocyanate (ANIT)[9,10], silica[11], carbon tetrachloride (CCl4)[12-14], 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)[15,16], and allyl alcohol (AA)[17-19]. This study mainly focused on the strain of FVB/N inbred mice, which have been widely used to generate a variety of transgenic lines owing to their high reproduction rate and easy access in microinjection[20,21].
Inbred FVB/N mice (8-10 wk old) were obtained from the National Laboratory Animal Center (Taipei, Taiwan). All animals received care as outlined in the “Guides for the Care and Use of Laboratory Animals” prepared by the Committee of Animal Research, Chang Gung Memorial Hospital. Mice were supplied with food and water and exposed to a 12-h light-dark cycle. Ten mice (five males and five females) composed a group to which surgical CBDL or a hepatotoxin via intraperitoneal (IP) or oral gavage (OG) administration at the same dosage was given during a period of 6 mo.
ANIT (Sigma Chemical Co., St. Louis, MO, USA) ANIT was dissolved in corn oil and administered via IP or OG route, respectively. For each route of administration, there were three subgroups according to the maintenance dose as follows: 25 mg/kg (low dosage), 50 mg/kg (medium dosage) and 125 mg/kg (high dosage). After a single loading dose of 100 mg/kg, the maintenance doses were given twice a week. Additionally, there was a modified method of OG administration with a single loading dose of 100 mg/kg and subsequent maintenance doses of 50 mg/kg for the 1st and 2nd mo, 75 mg/kg for the 3rd and 4th mo, and 100 mg/kg for the 5th and 6th mo.
AA (Sigma Chemical Co.) AA was prepared in double distilled water and administered via OG route for five subgroups according to the dosages as follows: 0.2, 0.4, 0.6, 0.8, and 1.0 mmol/kg twice a week.
CCl4 (Sigma Chemical Co.) The mice were fed via OG or IP route with CCl4 (density = 1.594 kg/L) diluted in double distilled water. As for each route of administration, they were further subgrouped into three according to the maintenance doses as follows: 0.8 g/kg (low dosage), 1.6 g/kg (medium dosage) and 2.0 g/kg (high dosage). After a single loading dose of 2.4 g/kg, the maintenance doses were then administered twice a week.
DDC (Sigma Chemical Co.) The mice were fed via OG with DDC in corn oil twice a week. They were subgrouped by four different dosages as follows: 0.25, 0.5, 2.5, and 5 mg. In addition, 0.1% DDC contained diet (Purina Mills TestDiet, St. Louis, MO, USA) was used as the 5th subgroup.
Silica (US Silica Company, Berkeley Springs, WV, USA) Silica was dissolved in Dulbecco’s modified eagle media and given subcutaneously at the dose of 3.5 g/kg, or intraperitoneally at the dose of 1.6 g/kg twice a week.
CBDL The surgical procedure was performed under sterile conditions. The mice were anesthetized with isoflurane. Midline laparotomy was performed for exploring the hepatic hilum and identifying CBD. Under the dissecting microscope, CBD was then isolated, doubly ligated and transected between two ligatures.
Liver biopsy demanded survival procedures and sterile techniques. After anesthesia with isoflurane, midline laparotomy was performed to expose the mouse liver. A liver block sized 0.5 cm in diameter was taken and then the cut surface was sutured for hemostasis. Serial biopsy was preformed monthly (per 4 wk) for each mouse till cirrhosis was well documented by histopathology. At the first liver biopsy, ascites, and intra-abdominal adhesion were examined and recorded.
Mouse livers from biopsies were fixed in 10% neutral buffered formaldehyde solution and embedded in paraffin. Sections, 4 µm in thickness, were stained with hematoxylin and eosin. Fatty change of hepatocytes was recorded when hepatocytes showed fat accumulation, balloon-like swelling or formation of fat cysts. For liver necrosis, there were the disorganization of liver cell cords, homogeneous cytoplasm, pyknotic nuclei and periportal infiltration of inflammatory cells. Fibrosis was defined as the presence of fibrous scarring band in the liver parenchyma by Mason’s trichrome collagen stain. Once the irregular zones of fibrosis developed and surrounded the remaining parenchymal tissues to display a pattern of nodularity under microscope, cirrhosis was diagnosed.
For survival and cirrhosis rate analyses, life table method was used. The length of time intervals used in this study was 4 wk. Any mouse which died, or whose liver was pathologically documented to be cirrhotic was considered to have experienced an event. When a mouse died before cirrhosis was confirmed at cirrhosis rate analysis, it was counted as a withdrawal and treated the same way as cases lost to follow-up. Plots were constructed with weeks after inducing liver injury vs survival or cirrhosis rate. The Wilcoxon test was employed to compare the curves. Differences were regarded as significant if a P value was less than 0.05.
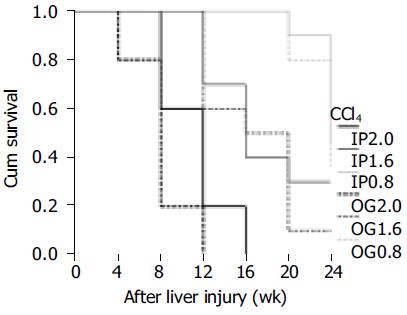
During the period of 6 mo, none of the 260 mice enrolled in this study died of the procedures for CBDL, liver biopsy or administration of hepatotoxins. There was no mortality case in the AA and silica groups. As for the other groups, 71 mice died in the course of this study. Among the 71 deaths, 20 occurred before cirrhosis could be histopathologically confirmed (pre-cirrhotic) and the remaining 51 after cirrhosis was found (post-cirrhotic). Nineteen of the 20 pre-cirrhotic deaths occurred in the CCl4 group. As for the post-cirrhotic deaths, there were 4 in the ANIT group, 26 in the CCl4 group, 11 in the DDC group and 10 in the CBDL group. The case numbers of mortality in each subgroup were listed in Table 1. Mortality had been found in all the CCl4 subgroups, ranging from 40% to 100%. In the DDC group, mortality was mainly post-cirrhotic, and seen in 2.5 and 5 mg and 0.1% diet subgroup. All the mice in CBDL group had post-cirrhotic death by the end of 16 wk. The overall comparison for the survival curves in the ANIT and DDC groups could not reach any statistical significance (P = 0.0823 and 0.1227, respectively), whereas there was a significant difference in the CCl4 subgroup (P < 0.0001). Pairwise comparisons for survival curves of the six CCl4 subgroups showed that the low dosage (IP0.8 and OG0.8) was significantly better than the medium dosage (IP1.6 and OG1.6, Figure 1, P = 0.0010-0.0317), and the medium dosage was also superior to the high dosage (IP2.0 and OG2.0, Figure 1, P = 0.0002-0.0044). Considering the administration routes, IP route compared favorably in survival with OG route at the high dosage (P = 0.0221), but not at the medium and low dosages (P = 0.7899 and 0.5902, respectively).
| Pre-cirrhotic death | Post-cirrhotic death | |
| ANIT OG 25 mg/kg | 0 | 0 |
| ANIT OG 50 mg/kg | 0 | 0 |
| ANIT OG 125 mg/kg | 0 | 3 |
| ANIT IP 25 mg/kg | 0 | 0 |
| ANIT IP 50 mg/kg | 0 | 0 |
| ANIT IP 125 mg/kg | 1 | 0 |
| ANIT modified | 0 | 1 |
| AA OG from 0.2 to 1.0 mmol/kg | 0 | 0 |
| CCl4 OG 0.8 g/kg | 0 | 5 |
| CCl4 OG 1.6 g/kg | 2 | 7 |
| CCl4 OG 2.0 g/kg | 5 | 5 |
| CCl4 IP 0.8 g/kg | 4 | 0 |
| CCl4 IP 1.6 g/kg | 6 | 1 |
| CCl4 IP 2.0 g/kg | 2 | 8 |
| DDC OG 0.25 mg | 0 | 0 |
| DDC OG 0. 5 mg | 0 | 1 |
| DDC OG 2.5 mg | 0 | 3 |
| DDC OG 5 mg | 0 | 4 |
| DDC 0.1% diet | 0 | 3 |
| Silica (SC 3.5 g/kg or IP 1.6 g/kg) | 0/0 | 0/0 |
| CBDL | 0 | 10 |
Ascites was universally found at laparotomy when the hepatotoxins were administered via IP route. In the CBDL group, there were only three mice presenting with ascites at the subsequent laparotomy. Intraabdominal adhesion existed in over 50 % of cases (ranging from 50% to 100%) when IP route or CBDL was used. Additionally, granuloma-like masses were intraperitoneally identified in all the cases of silica IP subgroup and in nine cases of ANIT IP subgroups (two for 25 mg/kg, one for 50 mg/kg and six for 125 mg/kg).
The case numbers of histopathological findings in each subgroup were listed in Table 2. Fatty change was seen mainly in the ANIT and CCl4 groups, and sporadically in the AA group (1 for 0.8 mmol/kg and 2 for 1.0 mmol/kg). Bile lakes were the common findings in the DDC and CBDL groups. Liver necrosis and fibrosis were demonstrated almost in all the cases of the ANIT, CCl4, DDC, and CBDL groups, two cases of the AA group (one for 0.8 mmol/kg and the other for 1.0 mmol/kg), but none of the silica group. The necrosis and fibrosis were detected at the first or second liver biopsy (4-8 wk after liver injury). Liver fibrosis in the two mice from the AA group did not progress to cirrhosis by the end of this study. Cirrhotic nodules could be identified in 30 mice (42.9%) of ANIT group (n = 70), 32 mice (53.3%) of CCl4 group (n = 60), 39 mice (78.0%) of DDC group (n = 50) and all the mice (100%) of CBDL group (n = 10). DDC subgroup with higher dosages (2.5 and 5 mg) and 0.1% diet could substantially reach 100% successful rate of cirrhosis induction as seen in the CBDL group.
| Fatty change | Necrosis/fibrosis | Cirrhosis | Bile lake | |
| ANIT OG 25 mg/kg | 0 | 10 | 2 | 0 |
| ANIT OG 50 mg/kg | 4 | 10 | 4 | 0 |
| ANIT OG 125 mg/kg | 5 | 10 | 8 | 0 |
| ANIT IP 25 mg/kg | 0 | 9 | 1 | 0 |
| ANIT IP 50 mg/kg | 1 | 10 | 1 | 0 |
| ANIT IP 125 mg/kg | 4 | 10 | 9 | 0 |
| ANIT modified | 4 | 10 | 5 | 0 |
| AA OG from 0.2 to 1.0 mmol/kg | 31 | 22 | 0 | 0 |
| CCl4 OG 0.8 g/kg | 4 | 10 | 5 | 0 |
| CCl4 OG 1.6 g/kg | 5 | 10 | 8 | 0 |
| CCl4 OG 2.0 g/kg | 8 | 10 | 5 | 0 |
| CCl4 IP 0.8 g/kg | 3 | 10 | 2 | 0 |
| CCl4 IP 1.6 g/kg | 5 | 10 | 4 | 0 |
| CCl4 IP 2.0 g/kg | 6 | 10 | 8 | 0 |
| DDC OG 0.25 mg | 0 | 10 | 3 | 6 |
| DDC OG 0. 5 mg | 0 | 10 | 6 | 9 |
| DDC OG 2.5 mg | 0 | 10 | 10 | 10 |
| DDC OG 5 mg | 0 | 10 | 10 | 10 |
| DDC 0.1% diet | 0 | 10 | 10 | 9 |
| Silica (SC 3.5 g/kg or IP 1.6 g/kg) | 0/0 | 0/0 | 0/0 | 0/0 |
| CBDL | 0 | 10 | 10 | 10 |
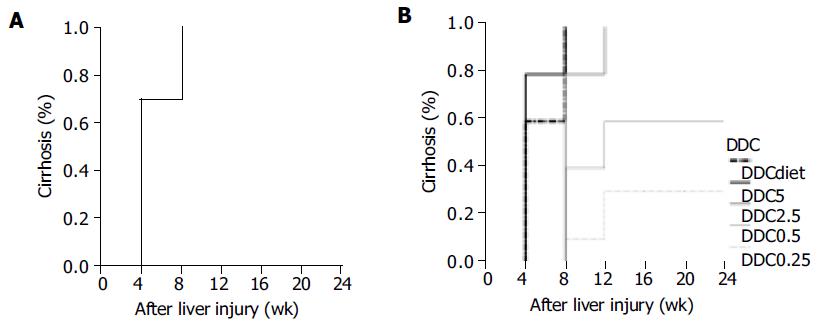
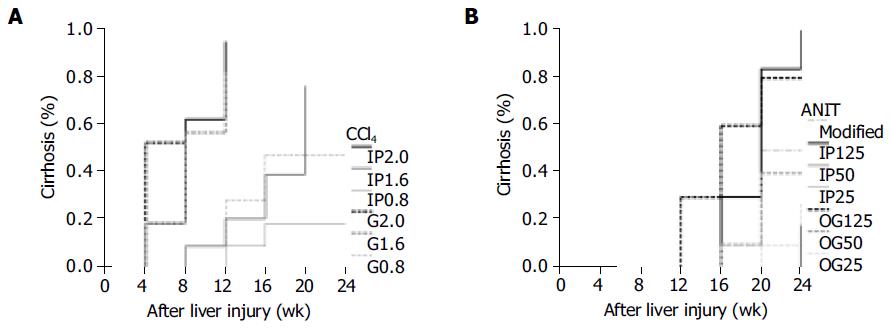
Results from life table analysis for the durations were presented in the curves with weeks after inducing liver injury vs cirrhosis rate. CBDL represented a rapid method to induce liver cirrhosis with 70-100% of successful rate within 4-8 wk (Figure 2A). DDC caused liver cirrhosis within 4-12 wk (Figure 2B). Statistical analysis revealed that the lower the DDC dosage, the longer the time spent by DDC in inducing cirrhosis among 0.5, 2.5 and 5 mg subgroups (DDC0.5 vs DDC2.5, P = 0.0387 and DDC2.5 vs DDC5, P = 0.0004). There was no significant difference between 0.25 and 0.5 mg DDC subgroups (P = 0.1311). DDC 0.1% diet required significantly less time to induce cirrhosis than DDC 0.25 mg (P = 0.0001), 0.5 mg (P = 0.0008) and 2.5 mg (P = 0.0032), but was not significantly different from 5 mg subgroups (P = 0.3415). As for CCl4, the duration for inducing cirrhosis was quite variable, ranging from 4 to 20 wk (Figure 3A). The IP and OG high dosages (2.0 g/kg) of CCl4 spent significantly less time in cirrhosis induction than IP and OG low dosages (0.8 g/kg) of CCl4 (P = 0.0005 - 0.0209). There was no difference of the cirrhosis curves between IP and OG admi-nistrations at the high (P = 0.1760) and low (P = 0.1661) dosages, whereas OG administration compared favorably in cirrhosis induction than IP administration at the medium dosage (1.6 g/kg) of CCl4 (P = 0.0060). The OG medium dosage (OG1.6) had no significant difference from the high dosage (P = 0.1852 for OG2.0 and P = 0.7241 for IP2.0), and the IP medium dosage (IP1.6) had no significant difference from the low dosage (P = 0.8822 for OG0.8 and P = 0.1514 for IP0.8). Cirrhosis caused by ANIT did not show up until 12-24 wk (Figure 3B). The high dosage of ANIT significantly spent less time in cirrhosis induction than the medium (P = 0.0002-0.0263) and low dosages (P = 0.0002-0.0027), whereas the medium dosage could not be significantly superior to the low dosage (P = 0.0904-1.0000). At the same ANIT dosage used, there was no significant difference of cirrhosis curves between OG and IP administrations (P = 0.2444 for high dosage, P = 0.0904 for medium dosage, and P = 0.5032 for low dosage). The modified method for ANIT administration compared favorably in cirrhosis induction only with IP low dosage and IP medium dosage (P = 0.0349, for both), but poorly with OG high dosage (P = 0.0303).
In this 6-mo study of experimental cirrhosis induction, AA and silica were ineffective in inducing liver fibrosis let alone cirrhosis. AA administration in the drinking water (50 ppm equivalent to 0.08-0.11 mmol/kg per d) for 15 wk has no effect on the histopathological examination of rat livers[19]. Moreover, IP injection of AA in rats only produced variable periportal liver necrosis predominantly at 6-12 h, followed by restitutive proliferation of periportal necrosis to repopulate the necrotic zone. Eventually, the liver architecture was essentially restored by 1 wk[18]. It indicated that AA merely induced short-term toxicity in rats. Although we had observed liver necrosis and fibrosis in two mice with OG administration of 0.8 and 1.0 mmol/kg AA, this liver damage was transient and insufficient to result in cirrhosis. It suggested that rats and FVB/N mice had similar hepatotoxic resistance to AA. As for silica, subcutaneous or IP administration was reported to cause liver fibrosis and cirrhosis in nude mice at 12 mo and in (C57BL/6×BALB/c) F1 mice at 18 mo[22]. Thus, a 6-mo duration might not be long enough for silica to cause hepatic injury with subsequent fibrosis and cirrhosis in FVB/N mice. As a result, a longer-term administration of silica might be required for inducing liver cirrhosis in FVB/N mice. A major drawback with silica administration was the granuloma formation at the injection site. This might be of concern to subsequent laparotomy for liver biopsy when the IP route is used.
The IP or OG administration of ANIT, CCl4 or DDC could effectively lead to liver fibrosis within 4-8 wk. All the liver fibrosis in mice did not always progress to microscopic cirrhotic nodules by the end of 24 wk. It suggested that restitutive response of mice to the injuries by those hepatotoxins sometimes restore liver architecture. ANIT has been used for years to study cholangiolitic hepatotoxicity in laboratory animals[9,10]. This study showed that ANIT usually led to mouse cirrhosis within 12-24 wk after administration. Mortality was only seen in the subgroups with high dosage and modified administration. However, there was no significant difference among the survival curves of all the ANIT subgroups. The high dosage of ANIT could more rapidly induce liver cirrhosis than the medium or low dosage. At the same dosage of ANIT, it made no significant difference between IP and OG administrations in cirrhosis induction. The modified method by gradual increase of ANIT dosage could not substantially provide any benefit in cirrhosis induction in this study. Overall, ANIT model required a longer period for the formation of cirrhosis. This might provide more time for researchers to investigate the progression of liver fibrosis.
CCl4 has been used extensively for decades to induce liver injury in various experimental models to elucidate the mechanisms behind hepatotoxicity[23]. Also, it was commonly used as a hepatotoxic agent in transgenic mice to evaluate liver fibrosis and cirrhosis on the basis of the selective expression of some novel or altered genes[13,14,24]. We experienced that the major problem in association with the use of CCl4 to induce liver fibrosis or cirrhosis had been the high mortality rates of mice, accounting for 40-100 % in those subgroups. Animals might die before or after cirrhosis was found. The higher CCl4 dosage was associated with the worse survival curve. The duration required for cirrhosis induction by CCl4 was quite variable, ranging from 4 to 20 wk, mainly depending upon the dosage. The high dosage (2.0 g/kg) of CCl4 could rapidly cause liver cirrhosis within 4-12 wk, but led to poor survival. Under the circumstances, IP administration will be suggested since IP route posed better survival than OG route only at the high dosage of CCl4. The low dosage (0.8 g/kg) of CCl4 required 12-24 wk to successfully induce cirrhosis. At the low dosage, it was shown that the administration routes had no influence on the survival and the cirrhosis induction. At the medium dosage (1.6 g/kg) of CCl4, OG administration was more rapid to induce cirrhosis than IP administration, but did not significantly differ in survival curves from IP administration. Nevertheless, the high mortality rate along with the use of CCl4 must be taken into consideration since it will prematurely terminate the sequential studies or observations of liver fibrosis or cirrhosis. Furthermore, CCl4 might not be an ideal solvent for investigating hepatocarcinoma since liver carcinogenesis might be due to the genotoxic chemical carcinogen effect of CCl4 itself as well as CCl4-induced liver cirrhosis[25,26].
DDC did not result in pre-cirrhotic death, but tended to lead to post-cirrhotic death at higher dosage (2.5 and 5 mg) and 0.1% DDC diet. The overall comparison for all survival curves of those DDC subgroups led to the insignificant difference. It took about 4-12 wk for DDC to successfully induce cirrhosis. Except 0.25 mg DDC subgroup, the successful rate of cirrhosis induction was quite satisfactory. The higher the dosage of DDC we used, the lesser the time we needed to induce cirrhosis. DDC 0.1% diet could not be superior to 5 mg DDC subgroup in cirrhosis induction. A major concern of DDC use was the high proportion of bile lake formation. This might cause some biases in observing the relationship between cirrhosis and its sequelae.
CBDL represented a rapid and consistent method to induce liver fibrosis and cirrhosis. Hepatocellular injuries were caused by cholestasis, leading to liver dysfunction, promoting fibrogenesis and ultimately resulting in liver failure and death. All the mice with CBDL rapidly progressed to cirrhosis within 4-8 wk after operation, and died by the end of 16 wk. The rapid progression to cirrhosis and death by CBDL might limit its application in chronic fibrosis studies and make it difficult to carry out a long-term investigation of liver cirrhosis. A major technical problem was the reversibility of histological changes due to recanalization of bile ducts[1]. In our tested animals, the bile ducts remained totally obstructed till their death in view of bile lakes at final pathology and the coloration of peritoneum and auricle. The successful obstruction by CBDL might be due to the fact that CBD was doubly ligated and transected between two ligatures[27]. Besides, intraabdominal adhesion could be an obstacle for subsequent survival laparatomy to study CBDL livers.
In summary, CBDL and hepatotoxins of ANIT, CCl4 and DDC could be effective in causing liver fibrosis of mice. CBDL rapidly and irreversibly led to liver fibrosis and subsequently to cirrhosis within 4-8 wk, but inevitably caused mortality by the end of 16 wk. As a result, CBDL was suitable for creating a mouse model for the short-term study of liver fibrogenesis. Although CCl4 had been commonly used for inducing liver fibrosis and cirrhosis, the duration required for cirrhosis induction varied (4-20 wk) and mainly depended upon the dosage. It could be used in mice for studying both acute and chronic liver fibrogenesis. A major drawback in association with CCl4 use was a relatively high mortality. DDC could effectively induce cirrhosis within 4-12 wk. The time required for cirrhosis induction by DDC was quite similar to that of CBDL, but the survival experience was better in the DDC group. ANIT slowly induced liver cirrhosis in mice. The duration from initial liver inflammatory damage to cirrhosis formation by ANIT was more than 12 wk after administration. Thus, ANIT was quite a good toxin for a long-term study of fibrosis progression into cirrhosis.
We thank Dr. Yun-Fan Liaw for his suggestions, helpful discussions and support.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Wu J, Norton PA. Animal models of liver fibrosis. Scand J Gastroenterol. 1996;31:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 485] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Hanahan D. Transgenic mice as probes into complex systems. Science. 1989;246:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 230] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Quick DJ, Shuler ML. Use of in vitro data for construction of a physiologically based pharmacokinetic model for naphthalene in rats and mice to probe species differences. Biotechnol Prog. 1999;15:540-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Lindstrom AB, Yeowell-O'Connell K, Waidyanatha S, McDonald TA, Golding BT, Rappaport SM. Formation of hemoglobin and albumin adducts of benzene oxide in mouse, rat, and human blood. Chem Res Toxicol. 1998;11:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Cunningham ML, Bucher JR. Pharmacodynamic responses of F344 rats to the mouse hepatocarcinogen oxazepam in a 90-day feed study. Toxicol Appl Pharmacol. 1998;149:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Watt KC, Buckpitt AR. Species differences in the regio- and stereoselectivity of 1-nitronaphthalene metabolism. Drug Metab Dispos. 2000;28:376-378. [PubMed] |
| 8. | Dill JA, Lee KM, Bates DJ, Anderson DJ, Johnson RE, Chou BJ, Burka LT, Roycroft JH. Toxicokinetics of inhaled 2-butoxyethanol and its major metabolite, 2-butoxyacetic acid, in F344 rats and B6C3F1 mice. Toxicol Appl Pharmacol. 1998;153:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Palmeira CM, Ferreira FM, Rolo AP, Oliveira PJ, Santos MS, Moreno AJ, Cipriano MA, Martins MI, Seiça R. Histological changes and impairment of liver mitochondrial bioenergetics after long-term treatment with alpha-naphthyl-isothiocyanate (ANIT). Toxicology. 2003;190:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ohta Y, Kongo M, Sasaki E, Harada N. Change in hepatic antioxidant defense system with liver injury development in rats with a single alpha-naphthylisothiocyanate intoxication. Toxicology. 1999;139:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Williams AO, Knapton AD. Hepatic silicosis, cirrhosis, and liver tumors in mice and hamsters: studies of transforming growth factor beta expression. Hepatology. 1996;23:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Natsume M, Tsuji H, Harada A, Akiyama M, Yano T, Ishikura H, Nakanishi I, Matsushima K, Kaneko S, Mukaida N. Attenuated liver fibrosis and depressed serum albumin levels in carbon tetrachloride-treated IL-6-deficient mice. J Leukoc Biol. 1999;66:601-608. [PubMed] |
| 13. | Brenner DA, Veloz L, Jaenisch R, Alcorn JM. Stimulation of the collagen alpha 1 (I) endogenous gene and transgene in carbon tetrachloride-induced hepatic fibrosis. Hepatology. 1993;17:287-292. [PubMed] |
| 14. | Inagaki Y, Truter S, Bou-Gharios G, Garrett LA, de Crombrugghe B, Nemoto T, Greenwel P. Activation of Proalpha2(I) collagen promoter during hepatic fibrogenesis in transgenic mice. Biochem Biophys Res Commun. 1998;250:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103-109. [PubMed] |
| 16. | Fickert P, Trauner M, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H. Bile acid-induced Mallory body formation in drug-primed mouse liver. Am J Pathol. 2002;161:2019-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Alam K, Nagi MN, Al-Shabanah OA, Al-Bekairi AM. Beneficial effect of nitric oxide synthase inhibitor on hepatotoxicity induced by allyl alcohol. J Biochem Mol Toxicol. 2001;15:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Yavorkovsky L, Lai E, Ilic Z, Sell S. Participation of small intraportal stem cells in the restitutive response of the liver to periportal necrosis induced by allyl alcohol. Hepatology. 1995;21:1702-1712. [PubMed] |
| 19. | Carpanini FM, Gaunt IF, Hardy J, Gangolli SD, Butterworth KR, Lloyd AG. Short-term toxicity of allyl alcohol in rats. Toxicology. 1978;9:29-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 443] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Perret-Gentil MI, Murray L, Bird DJ, Ladiges WC. Evaluation of FVB/N mice as recipients for transgenic embryos. Lab Anim Sci. 1999;49:427-428. [PubMed] |
| 22. | Ebbesen P. Chirality of quartz. Fibrosis and tumour development in dust inoculated mice. Eur J Cancer Prev. 1991;1:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Plaa GL. Chlorinated methanes and liver injury: highlights of the past 50 years. Annu Rev Pharmacol Toxicol. 2000;40:42-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Schnur J, Oláh J, Szepesi A, Nagy P, Thorgeirsson SS. Thioacetamide-induced hepatic fibrosis in transforming growth factor beta-1 transgenic mice. Eur J Gastroenterol Hepatol. 2004;16:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Zalatnai A, Lapis K. Simultaneous induction of liver cirrhosis and hepatocellular carcinomas in F-344 rats: establishment of a short hepatocarcinogenesis model. Exp Toxicol Pathol. 1994;46:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Beddowes EJ, Faux SP, Chipman JK. Chloroform, carbon tetrachloride and glutathione depletion induce secondary genotoxicity in liver cells via oxidative stress. Toxicology. 2003;187:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Prado IB, dos Santos MH, Lopasso FP, Iriya K, Laudanna AA. Cholestasis in a murine experimental model: lesions include hepatocyte ischemic necrosis. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |