Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3846
Revised: November 23, 2004
Accepted: November 26, 2004
Published online: July 7, 2005
AIM: To evaluate the expressions of apoptotic signal proteins FADD, TRADD, FasL, Fas, and NFκB in gastric carcinoma tissues and their clinical significance.
METHODS: Western blot immune trace method was adopted to detect the expressions of apoptotic signal proteins FADD, TRADD, FasL, Fas, and NFκB in 55 tissue specimens of gastric carcinoma.
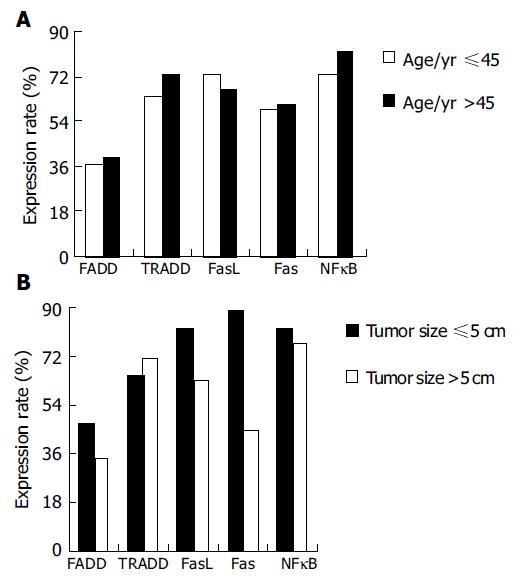
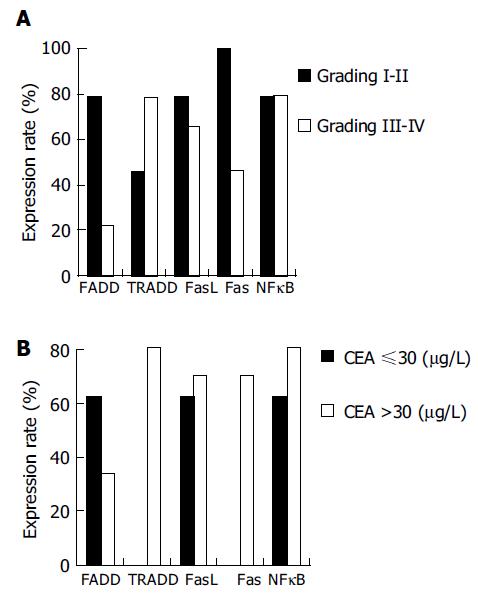
RESULTS: Five apoptotic signal proteins had different expressions in the gastric carcinoma samples and their expressions were not correlated to age (P = 0.085). Expressions of the FADD, FasL, Fas, and NFκB proteins reduced with increase of the volume of tumor with the exception of increased expression the TRADD protein (64.7-71.1%, P = 0.031). With gradual increase of the malignancy of gastric carcinoma tissues, expressions of the FADD, FasL, and Fas proteins decreased (78.6-28.0%, P = 0.008; 78.6-65.9%, P = 0.071; 100.0-46.3%, P = 0.014), while expressions of the TRADD and NFκB proteins increased (42.9-78.1%, P = 0.063; 78.6-79.1%, P = 0.134). With gradual increase of serum CEA, expression of the FADD protein decreased (62.5-34.0%, P = 0.073), but expressions of the TRADD, FasL, Fas, and NFκB proteins increased (0.0-80.8%, P = 0.005; 62.5-70.2%, P = 0.093; 0.0-70.2%, P = 0.003; 62.5-80.9%, P = 0.075). When compared to the tissues of gastric carcinoma without metastasis, the positive rate of expressions of the FADD and FasL proteins increased, whereas expressions of the TRADD, FADD, and NFκB proteins decreased. There was no significant difference between them (P = 0.095).
CONCLUSION: Gastric carcinoma is endurable to Fas-related apoptosis and apoptotic signal proteins are differently expressed in gastric carcinoma.
- Citation: Zhao XH, Gu SZ, Tian HG, Quan P, Pan BR. Clinical significance of expression of apoptotic signal proteins in gastric carcinoma tissue. World J Gastroenterol 2005; 11(25): 3846-3849
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3846
Gastric carcinoma is one of the most common causes of malignancy-related death in China[1-4]. Its therapy in clinics is a big challenge. Better remedial method possibly depends on advances in basic research[5-7]. Recent evidence suggests that apoptosis of cells is closely related to occurrence, progress and metastasis of the tumor[8-10]. At present, studies on the apoptosis of tumor cells are an important field of tumor therapy and tumor molecular biology[11-14]. The abnormalities in expressions of apoptotic signal proteins always influence the gastrocellular apoptosis, thus leading to the incidence and development of gastric carcinoma[15-17]. We adopted the Western blot immune trace method to detect the expressions of apoptotic signal proteins FADD, TRADD, FasL, Fas, and NFκB in gastric carcinoma tissues and their significance in gastric carcinoma was assessed.
A total of 55 cases were selected from patients with primary gastric carcinoma excision in our hospital from January 2000 to April 2001, who were pathologically diagnosed as adenocarcinoma. There were 35 male and 20 female cases aged 34-78 years with an average age of 54 years. According to Edmondson grading III, the samples were graded as I-II grade in 14 cases and III-IV grade in 41 cases. All samples were submerged in liquid nitrogen within half an hour after the excision and placed in a -80°C refrigerator.
Fifty-five tumor tissue specimens were homogenized, sonicated for 15 s twice in 500 µL of lysis buffer containing 1× phosphate-buffered saline (PBS), 1% Nonidet-P40, 0.5% sodium deoxycholate, 0.1% SDS and 0.1 mg/mL phenylmethylsulfonyl fluoride, and placed on ice for 30 min. The lysate was centrifuged at 13 000 g for 15 min at 4°C, the supernatant was collected (450 µL) and the protein concentration for each sample was determined by spectroph-otometry (Pharmacia) and using a DC protein assay kit (Bio-Rad).
Sheep antihuman FADD, TRADD, FasL, and Fas polyclonal antibodies, rabbit antihuman NFκB polyclonal antibodies, alkaline phophatase marking anti-sheep, anti-rabbit and protein molecular mass marker were also purchased from Santa Cruz Company. Western blot immune trace method was employed to determine the expression of apoptotic signal proteins. Twenty microliters of extracted protein for 120 g/L polyacrylamide gel electrophoresis. When it was semidry, electrical metastasis to the nitrocellulose film was made and enclosed with 100 mL/L bovine serum for 1 h. The first antibody (1:1 000) was put into PBST (1 g/L Tween20 in PBS) and stored overnight at 4°C. After being washed, the sample was enclosed again with 100 mL/L bovine serum for 30 min, and then the second antibody was put in at room temperature for 1 h. After being washed with PBS for 1 h, NBTBCIP development method was performed for coloration.
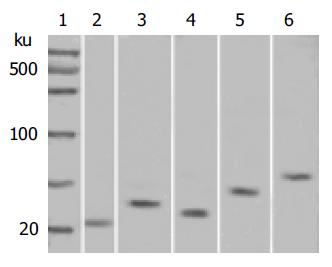
The protein molecular mass marker was taken as the standard. In the respective different molecular mass scope, the sample with black stripes was considered positive and vice versa negative. The molecular mass of FADD, TRADD, FasL, Fas, and NFκB proteins was 23, 34, 31, 48, and 65 ku respectively.
Chi squared test, accurate 4-chess table method and correlation analysis were used to analyze the correlation of protein expressions with sex, age, tumor size, pathological grading and tumor metastasis. P < 0.05 was considered statistically significant.
The expression of FADD, TRADD, FasL, Fas, and NFκB proteins in gastric carcinoma tissue specimens was 36.4% (20/55), 69.1% (38/55), 69.1% (38/55), 60.0% (33/55) and 78.2% (43/55) respectively (Table 1). We carried out Western blot analysis to determine the accuracy of protein expression (Figure 1).
| Sex | n | FADD | TRADD | FasL | Fas | NFκB |
| Male | 35 | 12 (34.3) | 26 (74.3) | 25 (71.4) | 21 (60.0) | 28 (80.0) |
| Female | 20 | 8 (40.0) | 12 (60.0) | 13 (65.0) | 12 (60.0) | 15 (75.0) |
| 55 | 20 (36.4) | 38 (69.1) | 38 (69.1) | 33 (60.0) | 43 (78.2) |
Different expressions of the five apoptotic signal proteins were not correlated to age (P = 0.085, Figure 2A). Expressions of the FADD, TRADD, FasL, Fas, and NFκB proteins reduced with increase of the tumor volume, with the exception of increased expression of the TRADD protein (64.7-71.1%, P = 0.031). Among them, the positive rate of the expression of the Fas protein in the tissue of gastric carcinoma with a lump ≤ 5 cm was higher than that of gastric carcinoma >5 cm and the difference was significant (P = 0.037, Table 2, Figure 2B).
| Clinical characteristics | n | FADD | TRADD | FasL | Fas | NFκB |
| Male | 35 | 12 (34.3) | 26 (74.3) | 25 (71.4) | 21 (60.0) | 28 (80.0) |
| Female | 20 | 8 (40.0) | 12 (60.0) | 13 (65.0) | 12 (60.0) | 15 (75.0) |
| Age/yr ≤ 45 | 22 | 8 (36.4) | 14 (63.6) | 16 (72.7) | 13 (59.1) | 16 (72.7) |
| > 45 | 33 | 13 (39.4) | 24 (72.7) | 22 (66.7) | 20 (60.6) | 27 (81.8) |
| Tumor | ||||||
| Size ≤ 5 cm | 17 | 8 (47.1) | 11 (64.7) | 14 (82.4) | 15 (88.2)a | 14 (82.4) |
| > 5 cm | 38 | 13 (34.2) | 27 (71.1) | 24 (63.2) | 17 (44.7) | 29 (76.3) |
| Grading I–II | 14 | 11 (78.6)b | 6 (42.9) | 11 (78.6) | 14 (100.0)c | 11 (78.6) |
| III–IV | 41 | 9 (22.0) | 32 (78.1) | 27 (65.9) | 19 (46.3) | 32 (78.1) |
| CEA ≤ 30 (µg/L) | 8 | 5 (62.5) | 0 (0.0) | 5 (62.5) | 0 (0.0) | 5 (62.5) |
| > 30 (µg/L) | 47 | 16 (34.0) | 38 (80.9)d | 33 (70.2) | 33 (70.2)d | 38 (80.9) |
| Tumor | ||||||
| transformation Yes | 6 | 3 (50.0) | 3 (50.0) | 5 (83.3) | 3 (50.0) | 3 (50.0) |
| No | 49 | 17 (34.2) | 35 (71.4) | 33 (67.4) | 30 (61.2) | 39 (79.6) |
With the increased of malignancy grade in adenocarcinoma tissues, expressions of the FADD, FasL, and Fas proteins decreased, while expressions of the TRADD and NFκB proteins increased. Expressions of the FADD and Fas proteins were significantly different between the pathological grades (P = 0.008 and P = 0.014, Figure 3A). With the increase of serum CEA, expression of the FADD protein in gastric carcinoma tissues decreased, while expressions of the TRADD, FasL, Fas and NFκB proteins increased. Expression of the TRADD and Fas proteins were positively correlated with the serum CEA level (r = 0.700, P = 0.005) and the difference was very significant (P = 0.003, Figure 3B). When compared to the tissues of gastric carcinoma with metastasis, the positive expression rate of the FADD and FasL proteins increased, whereas expressions of the TRADD, FAS and NFκB proteins decreased. There was no significant difference between them (P = 0.095).
Apoptotic abnormality is considered as an important mechanism underlying the development of gastric carcinoma[18,19] and even more crucial than the reproduction of cells of out control[20,21]. The expression of apoptotic signal proteins play different roles in the death of gastric cancer cells. The current studies[22,23] have denoted the main mechanism of the interaction of the apoptotic signal proteins to conduct down the apoptotic signal. FasL is polymerized with its corresponding Fas receptors[24,25]. By activating the FADD and caspase family, the apoptosis of cancer cells ensues; and by activating TRADD, RIP is motivated, causing NFκB to be activated, thereby participating in gene transcription of the survival of cells. The balance between counter signals and paths eventually determines the survival or apoptosis of cells.
In this study, the expression of Fas protein was downregulated with increase of the gastric carcinoma volume and the malignancy degree and positively correlated with the expression of CEA. It was reported[26] that gastric cancer cells inhibit expression of the downregulated FasL, thus escaping Fas-related apoptosis and immune monitoring of the host to enable cancer cells to reproduce continuously and increase their malignancy degree. Expression of the FADD protein is downregulated with increase of the malignancy degree of gastric carcinoma[27]. Gastric cancer cells might through the expression of the downregulated FADD enable caspases 3 and 8 necessary for apoptosis of Fas-related cells to be devitalized, resulting in inhibition of the apoptosis of gastric cancer cells. If FADD is injected, such cells can become sensitive to Fas and induce Fas-related apoptosis, suggesting that the function of FADD is extremely crucial.
The TRADD protein is able to vitalize the NFκB transcription factor and prolong the survival of cells. In the present study, the TRADD protein expression was positively correlated with CEA only when CEA possessed the value of 30 μg/L, suggesting that both of them can be used as indexes to detect gastric carcinoma. However, this can be determined only after investigating a large size of samples[28-30]. As the change of expressions of apoptotic signal proteins is not much related to metastasis of gastric carcinoma, the abnormalities of the function of these proteins only contribute to the reproduction and rise of malignancy degree of gastric cancer cells, whereas metastasis of gastric carcinoma might correlate to other factors.
In summary, gastric carcinoma is a tumor endurable to Fas-related apoptosis. Apoptotic signal proteins are differently expressed in gastric carcinoma.
Co-first-authors: Shan-Zhi Gu
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Duan LX, Zhong DW, Hu FZ, Zhao H, Yang ZL, Yi WJ, Shu GS, Hua SW. Relationship between expression ofVEGF, Flt1, bFGF and P53and outcomein patients with gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 2004;12:546-549. [Cited in This Article: ] |
| 2. | Xia JG, Ding YB, Chen GY. Expression of tyrosine kinase Syk and its clinical significance in gastriccarcinoma. Shijie Huaren Xiaohua Zazhi. 2004;12:767-769. [Cited in This Article: ] |
| 3. | Fukui T, Matsui K, Kato H, Takao H, Sugiyama Y, Kunieda K, Saji S. Significance of apoptosis induced by tumor necrosis factor-alpha and/or interferon-gamma against human gastric cancer cell lines and the role of the p53 gene. Surg Today. 2003;33:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kojima N, Kunieda K, Matsui K, Kato H, Saji S. Evaluation of carcinoembryonic antigen mRNA in living, necrotic, and apoptotic gastric cancer cells by reverse transcriptase-polymerase chain reaction. Surg Today. 2003;33:839-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Igarashi A, Konno H, Tanaka T, Nakamura S, Sadzuka Y, Hirano T, Fujise Y. Liposomal photofrin enhances therapeutic efficacy of photodynamic therapy against the human gastric cancer. Toxicol Lett. 2003;145:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Kokura S, Yoshida N, Ueda M, Imamoto E, Ishikawa T, Takagi T, Naito Y, Okanoue T, Yoshikawa T. Hyperthermia enhances tumor necrosis factor alpha-induced apoptosis of a human gastric cancer cell line. Cancer Lett. 2003;201:89-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Huang HL, Wu BY, You WD, Shen MS, Wang WJ. Influence of dendritic cell infiltration on prognosis and biologic characteristics of progressing gastric cancer. Zhonghua ZhongLiu ZaZhi. 2003;25:468-471. [PubMed] [Cited in This Article: ] |
| 8. | Wu YQ, Wang MW, Wu BY, You WD, Zhu QF. Expression of apoptosis-related proteins and proliferating cell nuclearantigen during stomach canceration. Shijie Huaren Xiaohua Zazhi. 2004;12:770-773. [Cited in This Article: ] |
| 9. | Nakashima S, Hiraku Y, Tada-Oikawa S, Hishita T, Gabazza EC, Tamaki S, Imoto I, Adachi Y, Kawanishi S. Vacuolar H+-ATPase inhibitor induces apoptosis via lysosomal dysfunction in the human gastric cancer cell line MKN-1. J Biochem. 2003;134:359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Zhan N, Xiong YY, Lan J, Wang BC, Tian SF, Yu SP. [Relationship between Helicobacter pylori infection and expression of c-myc, Bcl-2, and Bax protein in different gastric mucosa lesions]. Ai Zheng. 2003;22:1034-1037. [PubMed] [Cited in This Article: ] |
| 11. | Jiang XH, Wong BC. Cyclooxygenase-2 inhibition and gastric cancer. Curr Pharm Des. 2003;9:2281-2288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Suzuki H, Masaoka T, Nomura S, Hoshino Y, Kurabayashi K, Minegishi Y, Suzuki M, Ishii H. Current consensus on the diagnosis and treatment of H. pylori-associated gastroduodenal disease. Keio J Med. 2003;52:163-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003;2:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Valbonesi P, Sartor G, Fabbri E. Characterization of cholinesterase activity in three bivalves inhabiting the North Adriatic sea and their possible use as sentinel organisms for biosurveillance programmes. Sci Total Environ. 2003;312:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Bjørling-Poulsen M, Seitz G, Guerra B, Issinger OG. The pro-apoptotic FAS-associated factor 1 is specifically reduced in human gastric carcinomas. Int J Oncol. 2003;23:1015-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ishii H, Zanesi N, Vecchione A, Trapasso F, Yendamuri S, Sarti M, Baffa R, During MJ, Huebner K, Fong LY. Regression of upper gastric cancer in mice by FHIT gene delivery. FASEB J. 2003;17:1768-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Hahm KB, Kim DH, Lee KM, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Joo HJ, Cho YK. Effect of long-term administration of rebamipide on Helicobacter pylori infection in mice. Aliment Pharmacol Ther. 2003;18 Suppl 1:24-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kim DH, Kim SW, Song YJ, Oh TY, Han SU, Kim YB, Joo HJ, Cho YK, Kim DY, Cho SW. Long-term evaluation of mice model infected with Helicobacter pylori: focus on gastric pathology including gastric cancer. Aliment Pharmacol Ther. 2003;18 Suppl 1:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Jeong JH, Park JS, Moon B, Kim MC, Kim JK, Lee S, Suh H, Kim ND, Kim JM, Park YC. Orphan nuclear receptor Nur77 translocates to mitochondria in the early phase of apoptosis induced by synthetic chenodeoxycholic acid derivatives in human stomach cancer cell line SNU-1. Ann N Y Acad Sci. 2003;1010:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, Suzuki N, Inoue M, Soma G, Nagasue N. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 2003;23:5015-5022. [PubMed] [Cited in This Article: ] |
| 22. | Zhao Y, Wu K, Yu Y, Li G. [Roles of ERK1/2 MAPK in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells]. Wei Sheng Yan Jiu. 2003;32:573-575. [PubMed] [Cited in This Article: ] |
| 23. | Miyachi K, Sasaki K, Onodera S, Taguchi T, Nagamachi M, Kaneko H, Sunagawa M. Correlation between survivin mRNA expression and lymph node metastasis in gastric cancer. Gastric Cancer. 2003;6:217-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Yamaguchi H, Tanaka F, Sadanaga N, Ohta M, Inoue H, Mori M. Stimulation of CD40 inhibits Fas- or chemotherapy-mediated apoptosis and increases cell motility in human gastric carcinoma cells. Int J Oncol. 2003;23:1697-1702. [PubMed] [Cited in This Article: ] |
| 25. | Li Z, Wang Z, Zhao Z, Zhang Y, Ke Y. Expression of Fas, FasL and IFN-gamma in gastric cancer. Beijing Da Xue Xue Bao. 2003;35:386-389. [PubMed] [Cited in This Article: ] |
| 26. | Lim SC. Fas-related apoptosis in gastric adenocarcinoma. Oncol Rep. 2003;10:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Katoh M, Katoh M. FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 2003;22:1375-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Dechant MJ, Fellenberg J, Scheuerpflug CG, Ewerbeck V, Debatin KM. Mutation analysis of the apoptotic "death-receptors" and the adaptors TRADD and FADD/MORT-1 in osteosarcoma tumor samples and osteosarcoma cell lines. Int J Cancer. 2004;109:661-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Jamieson NB, McMillan DC, Brown DJ, Wallace AM. Comparison of simple acid-ethanol precipitation with gel exclusion chromatography for measuring leptin binding in serum of normal subjects and cancer patients. Ann Clin Biochem. 2003;40:185-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | El Yazidi-Belkoura I, Adriaenssens E, Dollé L, Descamps S, Hondermarck H. Tumor necrosis factor receptor-associated death domain protein is involved in the neurotrophin receptor-mediated antiapoptotic activity of nerve growth factor in breast cancer cells. J Biol Chem. 2003;278:16952-16956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |