Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3800
Revised: October 22, 2004
Accepted: December 20, 2004
Published online: June 28, 2005
Pancreatic carcinoma has a poor prognosis and early detection is essential for potentially curative resection. Despite the wide array of diagnostic tools, preoperative detection of small pancreatic carcinomas remains difficult. We report a case of small pancreatic carcinoma of the head of pancreas with indeterminate findings on US, ERCP, MRI and EUS which was successfully diagnosed via fusion CT-PET. This case illustrates the utility of CT-PET in the diagnosis of patients with small pancreatic carcinoma with equivocal findings on conventional diagnostic modalities.
- Citation: Goh BKP, Tan YM, Chung YFA. Utility of fusion CT-PET in the diagnosis of small pancreatic carcinoma. World J Gastroenterol 2005; 11(24): 3800-3802
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3800.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3800
Carcinoma of the pancreas has a poor prognosis with less than 20% of affected patients alive 1 year after diagnosis[1]. Early detection is essential for potentially curative resection. However, the preoperative diagnosis of pancreatic carcinoma remains difficult even with the wide array of diagnostic modalities available such as abdominal ultrasound (US), computed tomography (CT), endoscopic retrograde cholangiopancreatography (ERCP), magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS). These modalities have two main limitations. Firstly, poor detection of small lesions less than 2 cm that are suitable for curative resection and secondly, differentiating malignancy from inflammation. In addition, previous manipulation and stenting of the biliary tree adds to diagnostic uncertainties. This diagnostic difficulty results in two types of adverse outcome: (1) failure to resect a malignant tumor due to the absence of a definitive preoperative diagnosis or tissue confirmation of malignancy or (2) aggressive resection of a benign pathology[2]. Thus, there is a need for newer and more accurate imaging modalities to improve the preoperative diagnosis of pancreatic carcinoma.
Fusion computed tomography-positron emission tomography (CT-PET) with 2-deoxy-2-[18F]fluoro-D-glucose is a novel imaging modality which utilizes the principle of selective 18FDG-uptake and retention by malignant cells[2]. Early studies have demonstrated the accuracy of this procedure in the diagnosis of pancreatic malignancy with results comparable or even superior to conventional methods[2,3]. However, experience with this modality is limited as it is only available in a small number of institutions due to its high cost and its role in the evaluation of suspected pancreatic carcinoma is thus not yet well defined[3]. We report a case of pancreatic carcinoma with indeterminate findings on US, ERCP, MRI, and EUS which was successfully diagnosed preoperatively via fusion CT-PET.
A 48-year-old Chinese female was admitted with fever, jaundice associated with tea-colored urine and pruritis and right hypochondrial discomfort of 1-wk duration. She had no steatorrhea or loss of weight. Clinical examination of the abdomen did not reveal any palpable masses. Laboratory investigations showed a leukocyte count of 12.34×109/L and serum amylase 70 U/L. The liver function test revealed total serum bilirubin, 162 μmol/L; serum alkaline phosphatase, 1242 U/L; aspartate transaminase, 55 U/L and alanine transaminase, 65 U/L. Blood cultures, hepatitis B and C serology were negative and the tumor marker carbohydrate antigen (CA) 19-9 was elevated at 314 U/mL (normal, 3.0-50.0). A provisional diagnosis of cholangitis was made and she was started on intravenous ceftriaxone. Abdominal US demonstrated a normal gall bladder with dilated intra and extrahepatic ducts. No biliary stones, no pancreatic or hepatic masses were visualized. ERCP performed demonstrated dilatation of the biliary tree but no definite stricture of the common bile duct (CBD). The pancreatic duct could not be visualized. The patient’s cholangitis resolved following successful stenting and drainage during ERCP.
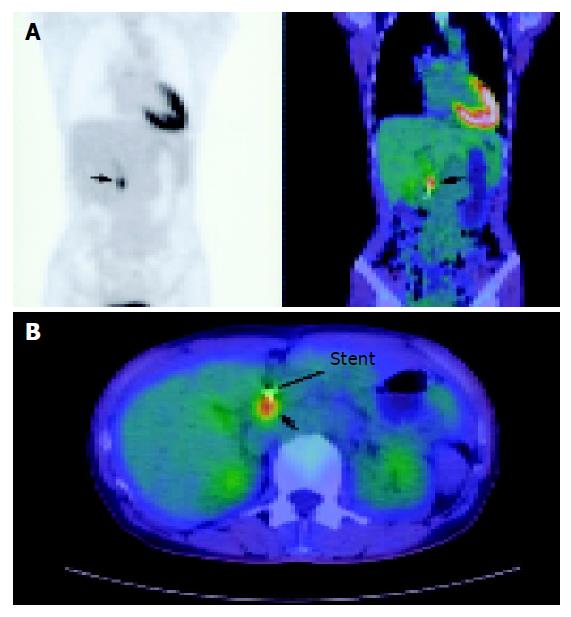
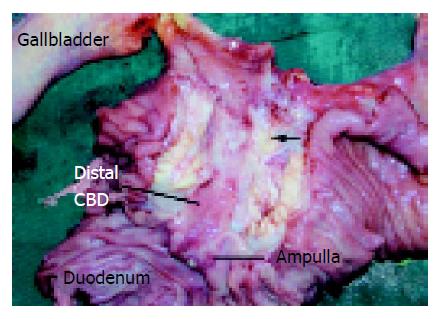
MRI of the pancreas and biliary tree was performed and this demonstrated dilatation of the biliary tree and pancreatic duct (double duct sign) suggestive of a pancreatic malignancy. However, no masses or lesions could be seen in the pancreas or distal CBD. The patient subsequently underwent EUS which also failed to demonstrate any definite nodules or masses in the pancreatic head. As the suspicion of a possible malignancy could not be excluded, CT-PET study was performed whereby 10.2 mCi of intravenous F-18 FDG was used as the tracer. This was followed by the attenuation corrected (by unenhanced CT) PET scan which was performed 60 min later. This demonstrated a focal metabolically active lesion related posterior to the stent in the distal CBD with a standard uptake value (SUV) of 7.0 compatible with carcinoma of the head of pancreas (Figures 1A and 1B). Based on the CT-PET findings, the patient underwent exploratory laparotomy whereby a 1.5-cm hard nodule was found within the head of pancreas. It invaded into the CBD, causing stricturing at the mid-level (Figure 2). The proximal CBD and main pancreatic duct were dilated and there were peripancreatic lymphadenopathy. Pancreatoduodenectomy was performed and final histology revealed a moderately differentiated adenocarcinoma of the head of pancreas invading into the duodenum with 1 of 17 lymph nodes involved. Her postoperative recovery was uneventful and she was discharged on the 8th postoperative day.
In 1931, Warburg observed that during the process of malignant transformation, neoplastic cells become avid glucose scavengers with increased glucose uptake, utilization and transport[2]. This enhanced glucose uptake and metabolism in malignant tissue provided the basis for the application of CT-PET scan. In pancreatic adenocarcinoma, a general increase in the expression of genes associated with the inward transport of glucose and glycolysis has also been demonstrated leading to its use as a diagnosis modality for pancreatic malignancy[2]. CT-PET has been reported to have a sensitivity from 82% to 100% and a specificity from 67% to 100% in the diagnosis of pancreatic carcinoma[2,4-7] and it is associated with a low false-positive rate of up to only 2.6%[5,6]. Notably, CT-PET has also been shown to be effective in differentiating carcinoma from chronic inflammation[1,8]. In the study by Rose et al, CT-PET was superior to CT in the diagnosis of pancreatic carcinoma with a sensitivity and specificity of 92% and 85% vs 65% and 62%, respectively. In the study of 18 patients (28%) with indeterminate or unrecognized pancreatic masses on CT were successfully diagnosed using CT-PET[2]. In another study by Diederichs et al, CT-PET was correct in 43 (84%) of 54 patients with indeterminate findings on CT or ERCP[9]. Thus, both these studies demonstrate the superiority of CT-PET over CT in the diagnosis of pancreatic carcinoma.
However, there are potential limitations to the specificity of CT-PET including its use in patients with previous upper gastrointestinal surgery, patients with an acute exacerbation of chronic pancreatitis, patients with pancreatitis related complications such as intracystic hemorrhage which can lead to nonspecific FDG accumulation and patients who have undergone recent interventional procedures (stent, probe placement)[1]. In particular, patients with an acute exacerbation of chronic pancreatitis may have an increased SUV with ranges similar to those for patients with pancreatic carcinoma[6]. Thus, serum amylase and lipase concentrations should be performed to exclude an acute exacerbation. Furthermore, there is evidence to suggest that diabetes mellitus may lead to false-negative results as the values of tumor uptake of FDG are lower in insulin-dependant diabetes patients compared with non-diabetics[6,8].
Current radiological imaging techniques are invaluable in the early diagnosis of pancreatic carcinoma. Abdominal US, CT and ERCP are the most commonly used imaging modalities for the evaluation of pancreatic pathologies and are widely regarded as ‘standard’ procedures in the diagnosis of pancreatic carcinoma[1]. However, MRI and more recently, EUS and PET are increasing rapidly as adjunctive diagnostic modalities.
Abdominal US is the most widely used imaging modality in patients with suspected pancreatic malignancy although its use is limited by a low sensitivity, operator dependence and high percentage of inadequate results[1]. ERCP has an accuracy of 90% and was once considered as the main diagnostic test[10,11]. However, there are several limitations and disadvantages of this diagnostic modality. Lesions not arising from the main ductal system may result in false-negatives and inflammatory pseudotumors may mimick carcinoma. Furthermore, ERCP is technically difficult with a lengthy learning curve and is associated with a failure rate of 3-10%[11]. It is also important to note that ERCP is an invasive procedure which may result in significant morbidity such as pancreatitis (1-8%) and mortality (0.2%)[9,12]. CT scan is usually performed in addition to ERCP to determine the size and extent of the tumor and detect distant metastasis. The sensitivity of CT in the diagnosis of pancreatic carcinoma is in the range of 50-90% and it is based on the size of pancreas, changes in contour, obliteration of peripancreatic tissue or other signs of invasive or metastatic disease[1,12]. With the use of the bolus technique with intravenous contrast medium and spiral CT, small tumors that do not produce a visible mass or alterations in the contour of the pancreas may be detected as focal areas with diminished enhancement[3,13]. However, the differentiation of mass-forming-pancreatitis from carcinoma is extremely difficult via CT scan.
MRI is fast replacing ERCP as the diagnostic procedure of choice as it is not only non-invasive, but also superior in that in addition to delineating the ductal system it allows visualization of masses in the pancreatic parenchyma as in CT. Furthermore, MRI has been shown to be superior to CT in the determination of local tumor extension[3]. The use of ERCP may thus be limited in the future to cases whereby MRCP findings are equivocal or when interventional procedures such as stenting needs to be performed. The use of EUS in the diagnosis has increased over the past decade and it has shown to be more sensitive than CT or MRI in detecting small lesions in the pancreas[14]. It can also localize lymph node metastases and vascular tumor infiltration with a greater sensitivity[14]. In addition, EUS allows fine needle biopsy of masses suspicious of malignancy with a sensitivity of 93% and specificity of 100%. Despite its advantages, EUS is associated with major limitations such as operator dependence and its limited field of visualization for detecting distant metastases; thus, limiting its use as an adjunct diagnostic modality. Furthermore, although it has a high sensitivity in detecting small lesions in the head of the pancreas, it is non-specific and may misdiagnose pseudo-tumors secondary to inflammation as malignancies.
Despite these wide-array of diagnostic modalities available in the armamentarium of the modern-day clinics, the preoperative diagnosis of pancreatic carcinoma remains difficult. A diagnostic problem that has remained unsolved is the differential diagnosis between focal or diffuse chronic pancreatitis and small pancreatic carcinoma. This case highlights this problem whereby chronic pancreatitis could not be excluded prior to the fusion CT-PET scan. None of the imaging modalities including US, CT, MRI or EUS was successful in demonstrating a pancreatic mass in this patient. Thus, although ERCP and MRI demonstrated a dilated biliary tree and a dilated pancreatic duct, a conclusive diagnosis of a pancreatic malignancy could not be made. This is because with the absence of a discrete pancreatic mass, other causes of the ‘double duct sign’ including concrements and chronic pancreatitis[11] could not be excluded. Nonetheless, we overcame the diagnostic difficulty in this patient where no discrete mass lesion could be identified with the use of fusion CT-PET. Fusion CT-PET demonstrated a focal metabolically active region in the head of pancreas with a SUV of 7.0. This was well above the cut-off for malignancy in previous studies (1.5-4.0)[1] thus confirming the diagnosis of pancreatic carcinoma.
In conclusion, this case illustrates the utility of fusion CT-PET in the preoperative diagnosis of pancreatic carcinoma in selected patients when the findings on more ‘conventional’ imaging modalities are indeterminate. All clinicians should be aware of the valuable role of this new imaging modality for the diagnosis of small pancreatic cancers not visualized on conventional imaging as early diagnosis and prompt treatment can affect long-term outcome.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Friess H, Langhans J, Ebert M, Beger HG, Stollfuss J, Reske SN, Büchler MW. Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut. 1995;36:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Rose DM, Delbeke D, Beauchamp RD, Chapman WC, Sandler MP, Sharp KW, Richards WO, Wright JK, Frexes ME, Pinson CW. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surg. 1999;229:729-737; discussion 737-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Balci NC, Semelka RC. Radiologic diagnosis and staging of pancreatic ductal adenocarcinoma. Eur J Radiol. 2001;38:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Berberat P, Friess H, Kashiwagi M, Beger HG, Büchler MW. Diagnosis and staging of pancreatic cancer by positron emission tomography. World J Surg. 1999;23:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Sperti C, Pasquali C, Chierichetti F, Liessi G, Ferlin G, Pedrazzoli S. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann Surg. 2001;234:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Imdahl A, Nitzsche E, Krautmann F, Högerle S, Boos S, Einert A, Sontheimer J, Farthmann EH. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg. 1999;86:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Clark L, Perez-Tamayo RA, Hurwitz H, Branch S, Baillie J, Jowell P, Coleman E, Pappas T, Keogan M, Tyler D. The role of positron emission tomography in the diagnosis and staging of pancreatic cancer. Gastroenterology. 1998;114:S0044. |
| 8. | Klever P, Bares R, Fass J, Büll U, Schumpelick V. PET with fluorine-18 deoxyglucose for pancreatic disease. Lancet. 1992;340:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, Beger HG, Reske SN. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1015] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 11. | Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg. 1990;159:59-64; discussion 64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Bilbao MK, Dotter CT, Lee TG, Katon RM. Complications of endoscopic retrograde cholangiopancreatography (ERCP). A study of 10,000 cases. Gastroenterology. 1976;70:314-320. [PubMed] |
| 13. | Raptopoulos V, Schellinger D. Imaging of the pancreas with computed tomography. Comput Tomogr. 1979;3:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Keogan MT, McDermott VG, Paulson EK, Sheafor DH, Frederick MG, de Long DM, Nelson RC. Pancreatic malignancy: effect of dual-phase helical CT in tumor detection and vascular opacification. Radiology. 1997;205:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |