Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3691
Revised: July 29, 2004
Accepted: September 4, 2004
Published online: June 28, 2005
AIM: To observe the therapeutic effect of intrasplenic transplantation with embryonic hepatocytes on amelioration of hereditary copper accumulation in toxic milk (TX) mouse modeling Wilson disease.
METHODS: Donor hepatocytes were harvested from 14-d fetal liver of a pregnant homogeneous DL mouse. These cells were successively cultured, labeled with fluorescein dye Hoechst 33342 for 24 h, and sequentially infused into the spleen parenchyma of the recipient TX mice. No host immunosuppression measures were taken. Two and four weeks after transplantation, the recipients were killed for routine histologic investigation and immunohistochemistry study up to 4 wk after transplantation. The serum copper and ceruloplasmin concentrations of the recipient mice were determined by graphite furnace atomic absorption spectroscopy.
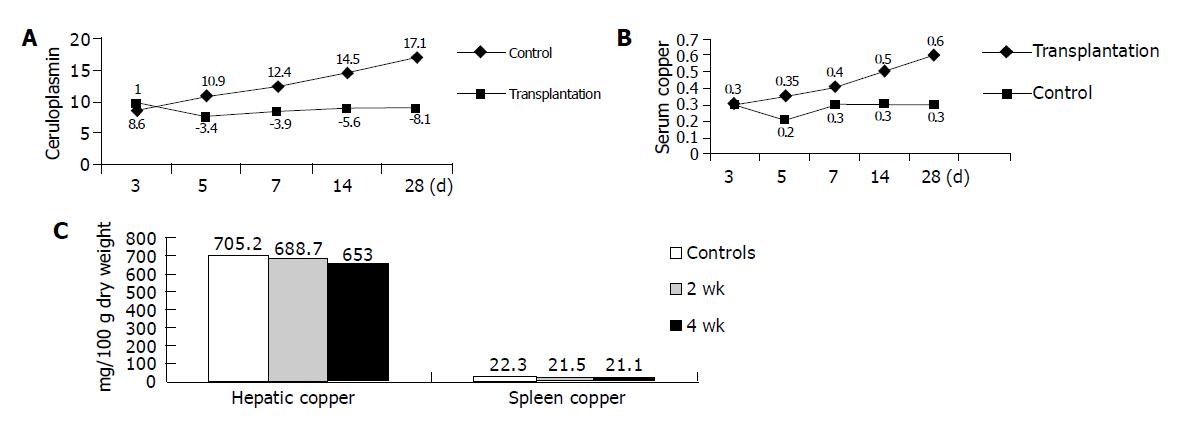
RESULTS: In the following 2nd and 4th wk after transplantation, the donor hepatocytes could be visualized in the livers of 47.3% recipients. The serum ceruloplasmin and copper concentrations increased by 1.6-fold after 2 wk and 2.0-fold times after 4 wk respectively, which ultimately rose from about 30% of the normal level to nearly 60% (P<0.01). The hepatic copper concentration decreased 7.2%, 4 wk after transplantation. Pathologic examination showed that there were many actively proliferative hepatocyte precursor cells with specific embryonic hepatocyte marker AFP migrated into hepatic sinusoids of the recipients. A large number of cells carrying hepatocytes marker and albumin were observed in the recipient spleen tissues.
CONCLUSION: Embryonic hepatocytes are capable of differentiating into mature hepatocytes in vivo. After transplantation, the hereditary abnormalities of copper metabolism in TX mice could be corrected partially by intrasplenic transplantation of homogeneous embryonic hepatocytes.
- Citation: Shi Z, Liang XL, Lu BX, Pan SY, Chen X, Tang QQ, Wang Y, Huang F. Diminution of toxic copper accumulation in toxic milk mice modeling Wilson disease by embryonic hepatocyte intrasplenic transplantation. World J Gastroenterol 2005; 11(24): 3691-3695
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3691
Wilson disease (WD), also known as hepatolenticular degeneration, is an autosomal recessive inherited disease characterized by impaired biliary copper excretion. The gene responsible for WD has been cloned and located to 13q14.3, encoding a copper transporting P-type ATPase ATP7B, which functions to transport copper across hepatocyte membranes. The mutant WD gene, which has lost the normal function of excreting copper, will result in toxic accumulation of copper in liver. The manifestations of WD include low serum ceruloplasmin, liver cirrhosis, extra-pyramidal symptoms, kidney lesion and cornea K-F ring[1]. D-penicillamine, a copper-chelating agent, plays the most important role in current therapeutic strategy for WD. The lesser the damage caused by copper toxicity, the greater the recovery following therapy[2]. But D-penicillamine has a long list of side effects and toxicities, and must be maintained for life-long[3]. Consequently, it is necessary to explore a more favorable therapy.
Orthotopic liver transplantation (OLT) is a choice of treatment for WD, which not only supplies patients with an alternative healthy liver but also helps to ameliorate copper metabolism disturbance[4,5]. Unfortunately, the number of patients who have benefited from OLT is very limited, because of the shortage of healthy donor livers, expensive cost and high risks for operation and immunological rejection following OLT. Hepatocyte transplantation is considered as a bridge to OLT and can provide patients with some hepatic function supports[6]. Nevertheless, mature hepatocytes are fully differentiated cells and hard to survive the required time for transplantation. Besides, the treatment still requires donor livers and needs invasive surgical procedure. Recently, with tremendous progresses in the field of stem cell research, much attention has been paid to another attractive alternative, progenitor hepatocytes, especially embryonic hepatocytes[7].
Toxic milk (TX) mouse is accepted widely as a valid animal model of WD bearing a WD gene mutation of Met1356Val[8]. The characteristics of copper metabolism and liver histopathology of TX mouse are coincident with those of WD patients. The current study was performed to explore whether transplanted embryonic hepatocytes are able to integrate into the liver tissue of recipient TX mice, proliferate there and subsequently ameliorate copper metabolism disorder in TX mouse.
DL and homologous TX mice were kindly donated by Dr. Julian Mercer (Deakin University, Australia) and used respectively as hepatocyte donors and recipients. All animals were bred in Special Animal Experimental Center of Sun Yat-Sen University and maintained at 18-25 °C in a 12-h light/12-h dark cycle. They had free access to food and water.
Thirty TX mice (female:male = 1:1) were randomly assigned to two experimental groups, 20 mice in T group underwent embryonic hepatocytes transplantation, 10 mice in C group were used as control. Animals from each group were killed at 2 or 4 wk after transplantation for histologic examination of the liver and spleen.
The isolation procedure was based on collagenase digestion techniques and adapted for use with embryonic livers. The pregnant 14-d DL mouse was anesthetized and subjected to midline laparotomy. The fetuses were removed and the embryonic livers were chopped up, washed twice with Ca++ and Mg++ free PBS, and then incubated with 0.05% collagenase type I. The final suspension was incubated in DMEM/F12 culture medium with 5% FBS for 24 h at 37 °C, in 50 mL/L CO2. Then debris of tissue and cells were washed out and serial static culture was performed until monolayer cells covered about 80% of the bottom of the flask. The fluorescein dye Hoechst 33342 was added to a final concentration of 10 μg/mL. These cells were incubated for 8 h at 37 °C and then washed twice with PBS. Fluorescence of labeled cells was verified by fluorescent microscopy. Before transplantation the number and viability of cells were estimated by trypan blue exclusion.
Under pentobarbital anesthesia (25-30 mg/kg intraperitoneally), the peritoneal cavity of recipient was accessed through a midline laparotomy under sterile conditions. Transplantation was carried out by direct injection into the spleen pulp of two million hepatocytes suspended in 100 μL PBS for the transplantation group (n = 20). Control group (n = 10) received the same procedure except for the blank 100 μL PBS.
Transplanted animals were killed 2 or 4 wk after transplantation respectively. Left lateral liver lobe and spleen were dissected and processed for routine histopathologic examination using standard techniques. Paraffin embedded 5-μm sections were stained with hematoxylin-eosin. Several other parts of dissected organs were snap frozen at -70 °C, 10-μm cryostat was sectioned and examined by fluorescent microscopy to identify Hoechst 33342-labeled donor hepatocytes.
To investigate the distribution and differentiation process of grafted embryonic hepatocytes in spleen of recipient mice, immunohistochemical staining of the recipient spleen tissue was employed. Sections of paraformaldehyde-fixed tissue were deparaffinized in xylene and rehydrated in graded alcohol. Antigen retrieval was carried out by microwave citrate buffer method and digested by trypsin at 37 °C for 30 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol. The primary antibodies (rabbit polyclonal albumin antibodies from DAKO Company, goat polyclonal AFP antibodies from Santa Cruz Company) were diluted in PBS plus 0.2% non-fat dried milk and applied at 4 °C overnight. After rinsing, secondary antibodies (goat anti-rabbit IgG(AP) and rabbit anti-goat IgG(Cy3)) were added and incubated at room temperature for 1 h. For each antibody, negative controls were performed by omitting the primary antibody from the protocol.
Fresh liver samples were dehydrated overnight in a vacuum oven and solubilized in nitric acid immediately before analysis. Copper concentration was measured by graphite furnace atomic absorption spectroscopy. The serum ceruloplasmin level was determined by measuring the p-phenylenediamine dihydrochloride oxidase activity of ceruloplasmin. Age and sex-matched DL mice were used as normal controls.
The values were presented as mean±SD. Differences were analyzed with Student’s unpaired t test. P<0.05 was considered statistically significant.
Twenty-four hours after primary culture, most cell clusters attached to the substrates of plastic flask. Two days later, phase-contrast microscope revealed that lots of spherical or oval shaped cells migrated outside the mass. These cells were predominantly mononuclear, bearing a high nucleus-plasma ratio with an average diameter of about 10 μm. As a result of migration and proliferation, an outgrowth around the tissue mass came into shape with some epithelioid cells and fibroblasts. After incubation for 2-3 wk, embryonic hepatocytes grew into block and attached tightly to each other. Once the embryonic hepatocytes were subcultured, these cells would irreversibly differentiate into fully differen-tiated adult hepatocytes with typical epithelium morphology.
All animals survived following intrasplenic injection until they were killed. Under optical microscope, the hepatocytes of TX mouse liver arranged in coarse nodules and lost the normal structure of hepatocyte cords. Some parenchymal hepatocytes contained swollen cytoplasm and enlarged, vacuoles-like nuclei with rare lipid droplets and frequent diploid nuclei. Another noteworthy characteristic was the extensive infiltration of lymphocytes in hepatic sinusoid. In animals treated with hepatocyte transplantation, although some monocytes infiltrated in the markedly altered lobule area, more diploid nuclei were observed. In addition, there appeared many more active proliferating oval cells with smaller nuclei, which were about one-fourth volume of the normal hepatocytes. No vessel distension, blockage or liver parenchyma necroses were observed.
To determine the distribution and proliferation of transplanted embryonic hepatocytes in recipient liver, we utilized Hoechst 33 342 to label donor cells. The animals with integrated appearance of engrafted modified hepatocytes in their liver sections were regarded as successful transplantation. Fluorescence microscopy revealed the presence of fluorescence-labeled donor cells 4 wk after transplantation in both liver and spleen sections. Two weeks after embryonic hepatocyte transplantation, a great deal of labeled embryonic hepatocytes with normal morphology distributed throughout the red pulp of the recipient spleen. In contrast, the number of fluorescent cells in liver parenchyma sections was small and only some isolated hepatocytes could be observed, and only occasionally 2 or 3 cells were aggregated together. These hepatocytes were found predominantly in small branches of the hepatic sinusoid. After 4 wk, many intrasplenically implanted cells migrated from spleen to liver through portal vein, concentrating in hepatic sinusoids. The donor cells in spleen still located in the red pulp with a few cells phagocytosed by the macrophages in spleen. In liver, marked donor cells appeared more frequently, and most of them aggregated into nodules containing 5-6 cells.
Copper and holoceruloplasmin levels were determined in plasma samples from all experimental mice in the following 4 wk after transplantation. On the 14th d after transplantation, 10 TX mice from T group were killed. Liver and spleen tissues and serum were taken for tissue and serum copper determination and holoceruloplasmin level assay. Donor cells with intact morphology were observed in 4 of 10 mice. On the 28th d, the other 10 mice were killed and successful transplantation was achieved in five mice. The copper concentrations and holoceruloplasmin activity values in all study groups are outlined in Figure 1.
The spleen sections were stained with antibodies to embryonic hepatocyte maker AFP and specific mature hepatocyte marker albumin to observe the differentiating process of engrafted embryonic hepatocytes in vivo. Although morphometric analysis was not performed, the number of immature hepatocytes carrying AFP decreased significantly 2 wk after transplantation. At the same time hepatocytes with specific mature hepatocyte marker albumin increased significantly.
Recently, the possibility to treat hepatic inborn errors of metabolism with hepatocyte transplantation rather than with whole liver transplantation has been demonstrated in more and more animal experiments and clinical trials, such as hereditary tyrosinemia type I, Crigler-Najjar syndrome, dextrinosis and 1a, and hereditary hypercholesterolemia[9-13]. Mature hepatocytes transplantation had been carried out to treat LEC rats, another WD animal model of inborn copper metabolic disturbance by Malhi et al[13]. Of the recipients, 9 rats out of all 14 rats were confirmed as successful repopulation by RT-PCR. The biological analysis also showed amelioration of copper metabolic disturbance. However, mature hepatocytes are highly differentiated somatic cells with limited capability of proliferating in vitro and immunogenicity. These disadvantages hindered the wide use of hepatocyte transplantation clinically.
In recent years, hepatic progenitor cells, also known as oval cells, are of interest in the field of liver cell transplantation and stem cell transplantation[14]. Some studies have demonstrated that oval cells in rats are bone marrow-derived cells and share some common cell surface markers with hematopoietic cells[15,16]. However, some experiments revealed that bone marrow-derived cells do not contribute significantly to oval cell or hepatocyte population in some specific setting[17]. Although the precise source of oval cells in mature liver needs to be investigated further, it has been widely accepted that there are a large amount of hepatic progenitor cells in embryonic liver. Embryonic hepatocytes originate from endodermal cells that form the so-called hepatic diverticulum detectable on d 10-12 after gestation on the ventral side of frontal endoderm. The process of embryonic hepatocyte differentiation is reversible. Hepatoblasts could become ductal cells that in turn differentiate into oval cells. Oval cells are undifferentiated cells that could give rise to mature hepatocytes or biliary cells, demonstrating high proliferative capacity[18]. Besides, embryonic hepatocytes possess greater resistance to chemical damage. Furthermore, these cells are still immature cells bearing incomplete MHC II surface antigen, thus possessing lower immunogenicity. Therefore, embryonic hepatocytes might be an ideal candidate donor cells for hepatocyte transplantation.
The current study addressed whether homogeneous engraftment of embryonic hepatocytes could be translated into greater liver population in vivo, and subsequently replaces the missing function of WD protein to excrete copper toxin from liver. Within 4 wk after transplantation, the donor embryonic hepatocytes initially transplanted into the recipient spleen could migrate to liver, colonize, proliferate there and develop into adult hepatocytes. Successful transplantation was achieved in 9 of 20 cases, accounting for 45%. Although fluorescence microscopy revealed that the labeled donor cells predominantly located in the red pulp of spleen, there were more labeled hepatocytes in liver parenchyma after 4 wks. Most of them aggregated into nodules containing 4-6 cells. The inborn copper metabolic disturbance was ameliorated in accordance to these pathologic changes. The level of serum ceruloplasmin and copper increased gradually and ultimately rose to about 60% of the normal level in syngeneic DL mice. Previous investigators favored the intrasplenic route of hepatocytes transplantation and suggested that transplanted hepatocytes could infuse into the portal vein and ultimately migrate to the hepatic sinusoid. When these cells accommodated the microenvironment, they proliferated there, integrated into the normal liver lobule structure and exert to correct the inborn errors of impaired copper excretion[19].
In our study, the ceruloplasmin activity began to rise from the 5th d after transplantation. Packman et al[20], studied the distribution of murine copper transporting ATPase during mouse embryonic development and reported that ATP7A (another copper transporting ATPase responsible for Menkes disease), but not ATP7B, is the predominant copper transporting factor in embryonic hepatocyte copper metabolism. In fetal liver, ATP7A does not involve in the synthesis of ceruloplasmin, but has the basic function to excrete copper. While ATP7B, playing the most important role in copper excretion of adults, gradually takes dominant position in postnatal liver development. Immunohistochemical staining in the present study revealed that some transplanted embryonic hepatocytes transplanted into the recipient spleen developed into mature hepatocytes bearing albumin, the typical marker of hepatocytes. It seems that the time for amelioration of ceruloplasmin might be in agreement with the time required for embryonic hepatocyte maturation. Therefore, these cells in recipient spleen contribute to the synthesis of ceruloplasmin.
Liver is the central organ of copper homeostasis. Under physiologic circumstances, bile excretion represents the sole mechanism for copper excretion and thus only those cells lodged in recipient liver have the practical ability to excrete copper. In the present study, the donor hepatocytes were present as small foci and seemed to grow by clonal population expansion within host parenchyma 4 wk after transplantation. To address whether these cells possess the ability to excrete copper into bile canaliculus, hepatic copper concentrations were evaluated. As expected, hepatic copper concentration decreased slightly 2 wk after transplantation, and showed a significant difference after 4 wk. It was reported that, shortly after transplantation, the transplanted cells become stacked at the portal vein radicles[14,21]. Although the majority of the donor cells are cleared, a portion of cells start to translocate into the space of Disse by disrupting the sinusoidal endothelium and finally join adjacent host hepatocytes. Therefore, transplanted embryonic hepatocytes might participate in the reconstitution of new functional biliary system and restore effective copper excretion.
This study showed that hereditary abnormalities of copper metabolism in TX mice could be corrected partially by intrasplenic transplantation of normal embryonic hepatocytes. Generally speaking, at least 15% of the liver parenchyma is needed to retain the physiological functions such as metabolism, coagulating and detoxification. What still needs to be investigated further include the number of engraft cells, the way of transplantation and long-term evaluation. Although the above data might be too preliminary for unambiguous conclusions, we believe that the accurate and long-term laboratory investigation, as well as clinical follow-up, will eventually benefit a broader use of embryonic hepatocyte transplantation in patients with WD.
The authors wish to thank Professor Ju Lian Mercer for donating TX and DL mice. The author also thanks colleagues and collaborators for providing information and discussion in this article.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Prashanth LK, Taly AB, Sinha S, Arunodaya GR, Swamy HS. Wilson's disease: diagnostic errors and clinical implications. J Neurol Neurosurg Psychiatry. 2004;75:907-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Jabłońska-Kaszewska I, Drobińska-Jurowiecka A, Dabrowska E, Trocha H. Results of treatment of Wilson's disease--own observations. Med Sci Monit. 2003;9 Suppl 3:9-14. [PubMed] |
| 3. | Ferenci P. Review article: diagnosis and current therapy of Wilson's disease. Aliment Pharmacol Ther. 2004;19:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Emre S, Atillasoy EO, Ozdemir S, Schilsky M, Rathna Varma CV, Thung SN, Sternlieb I, Guy SR, Sheiner PA, Schwartz ME. Orthotopic liver transplantation for Wilson's disease: a single-center experience. Transplantation. 2001;72:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Podgaetz E, Chan C. Liver transplantation for Wilson s disease: our experience with review of the literature. Ann Hepatol. 2003;2:131-134. [PubMed] |
| 6. | Hodgson H. Liver cells: biology to therapeutics. Clin Med. 2003;3:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Theophilos MB, Cox DW, Mercer JF. The toxic milk mouse is a murine model of Wilson disease. Hum Mol Genet. 1996;5:1619-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Grompe M. Liver repopulation for the treatment of metabolic diseases. J Inherit Metab Dis. 2001;24:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology. 2000;119:1720-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Mitchell C, Mignon A, Guidotti JE, Besnard S, Fabre M, Duverger N, Parlier D, Tedgui A, Kahn A, Gilgenkrantz H. Therapeutic liver repopulation in a mouse model of hypercholesterolemia. Hum Mol Genet. 2000;9:1597-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Malhi H, Irani AN, Volenberg I, Schilsky ML, Gupta S. Early cell transplantation in LEC rats modeling Wilson's disease eliminates hepatic copper with reversal of liver disease. Gastroenterology. 2002;122:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Sukhikh GT, Shtil AA. Stem cell transplantation for treatment of liver diseases: from biological foundations to clinical experience (review). Int J Mol Med. 2003;11:395-400. [PubMed] |
| 15. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 16. | Wang J, Clark JB, Rhee GS, Fair JH, Reid LM, Gerber DA. Proliferation and hepatic differentiation of adult-derived progenitor cells. Cells Tissues Organs. 2003;173:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11881-11888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 304] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Gupta S, Rajvanshi P, Irani AN, Palestro CJ, Bhargava KK. Integration and proliferation of transplanted cells in hepatic parenchyma following D-galactosamine-induced acute injury in F344 rats. J Pathol. 2000;190:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Notenboom RG, van den Bergh Weerman MA, Dingemans KP, Vermeulen JL, van den Eijnde S, Reutelingsperger CP, Hut H, Willemsen R, Offerhaus GJ, Lamers WH. Timing and sequence of differentiation of embryonic rat hepatocytes along the biliary epithelial lineage. Hepatology. 2003;38:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Kuo YM, Gitschier J, Packman S. Developmental expression of the mouse mottled and toxic milk genes suggests distinct functions for the Menkes and Wilson disease copper transporters. Hum Mol Genet. 1997;6:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Malhi H, Gupta S. Hepatocyte transplantation: new horizons and challenges. J Hepatobiliary Pancreat Surg. 2001;8:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |