Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3665
Revised: February 25, 2005
Accepted: May 13, 2005
Published online: June 28, 2005
AIM: To investigate the bile acid pool size after cholecystectomy whether or not correlated to the gastrointestinal migrating myoelectric complex (MMC) in guinea pigs.
METHODS: Gallbladder motilities were assessed before cholecystectomy. Furthermore, we continuously monitored interdigestive gastrointestinal motilities using bipolar electrodes in conscious guinea pigs before and after surgery at 4 wk in standard diet group and high cholesterol diet (cholesterol gallstone) group. Total bile acid pool sizes were measured by isotope dilution method at meantime.
RESULTS: After cholecystectomy, there were parallel falls in duration of phase I, II, III and MMC cycle duration but increase in amplitude in the guinea pigs with normal gallbladder function, and in the guinea pigs with cholesterol stones. However, There were not significantly differences. On the other hand, the bile acid pool was definitely small in the GS guinea pigs compared to normal guinea pigs and became slightly smaller after cholecystectomy. Similarly, bile acid in gallbladder bile, fecal bile acid was slightly increased in GS guinea pigs after cholecystectomy, to the same degree as normal. These differences, however, were not significant.
CONCLUSION: It is concluded that in the guinea pigs with normal gallbladder function, and in the guinea pigs with cholesterol stones: (1) Cholecystectomy produce a similar but less marked trend in bile acid pool; and (2) MMC are linked to enterohepatic circulation of bile acids, rather than surgery, which is consistent with changes of the bile acid pool size. As a result, gastrointestinal dyskinesia is not involved in occurrence of postchole-cystectomy syndrome.
- Citation: Zhang XM, Dong L, Liu LN, Chang BX, He Q, Li Q. Changes of gastrointestinal myoelectric activity and bile acid pool size after cholecystectomy in guinea pigs. World J Gastroenterol 2005; 11(24): 3665-3670
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3665
It is generally accepted that cholecystectomy as an alternative means to treat gallbladder diseases. However, recently more attention has been focused on removal of a gallbladder might result in change of enterohepatic circulation of bile acids (EHC) and whether or not there is abnormal of gastrointestinal myoelectric activity.
Interdigestive gastrointestinal motility is characterized by a typical cycling complex of myoelectric activity (MMC). Enterohepatic circulation of bile acids is essential for regular cycling of MMC. The bile acid pool may be defined as the total mass of bile acids in the enterohepatic circulation. Therefore, it is necessary to make clear relationship between bile acid pool size and MMC characteristics. However, because of technical difficulties in measuring of bile acid pool size in human being before cholecystectomy, the role of the bile acid pool has less investigated, the exact mechanism of change gastrointestinal myoelectric activity and relationship between MMC characteristics and bile acid pool size after cholecystectomy has not yet been clear.
The current study is conducted to investigate (1) the effects of cholecystectomy on changes of gastrointestinal myoelectric activity and bile acid pool size in normal and GS guinea pigs; (2) whether a correlation exists between interdigestive MMC and bile acid pool size.
[3H] Taurocholic acid (radiochemical purity >97%; specific activity 3.5Ci/mmol) was purchased from Perkin-Elmer Life Sciences (Boston, MA). Scintillation liquid was obtained from Sigma-Aldrich (St. Louis, MO).
Sixty female Hartley guinea pigs weighing 350-400 g were purchased from Animal Care Committee of Xi’an jiaotong University. Animals were randomly allocated to four groups (15 in each group). They were fed control diet and 1% (high) cholesterol diet (about 0.3 g/d) for 8 wks, respectively. There were not any differences in the diets apart from the cholesterol content in all groups. Two groups of guinea pigs (B group: half of the controls and D group: half of the cholesterols) were had surgical resection of the gallbladder performed as described below. Nor did the remaining two groups (A group and C group). There were no significant differences in weight gain or final weight of guinea pigs fed with the different diets. All guinea pigs were housed in a light cycle room (light from 7:00 AM to 7:00 PM) at 22 °C. Diet and water were provided ad libitum.
Gallbladder emptying was assessed by monitoring fasting and postprandial course of gallbladder volumes. The guinea pig gallbladder volume was estimated using a real-time ultrasonographic scanner equipped with a 7.5 MHz convex probe (Esaote-AU5 Italy). After an overnight fast, the fasting gallbladder volume (FV) was measured thrice within 5 min, with the guinea pigs lying supine or turned partially on their sides. Sagittal and transverse scans of the gallbladder at its largest dimension (maximum long diameter, L; maximum wide diameter, W; maximum high diameter, H) were obtained. Then, the residual gallbladder volume (RV) would not measured by the same method until 30 min after the guinea pigs were given a liquid fatty meal orally (2 mL of milk) containing protein 0.09 g, fat 0.1 g and carbohydrate 0.1 g, with a total of 1.70 kcal[1]. All measurements were carried out by the same ultrasonographer.
The fasting and residual gallbladder volumes were calculated as the mean of three values from frozen sonographs assuming a sum-of-ellipses method as described by Dodds et al[2].
V = π/6×(L×W×H)
Gallbladder ejection fraction (E) can be calculated from the following formula:
E = (FV-RV)/FV×100%
Animal experiments After being fasted for 12 h, guinea pigs underwent cholecystectomy via a 3 cm transverse incision 0.5 cm below the right costal margin under anesthesia (ketamine, 50 mg/kg; xylazine, 4 mg/kg, and acepromazine, 0.5 mg/kg, administered intramuscularly) in B group and D group. The peritoneal cavity was entered through a midline incision and four bipolar insulated silver electrodes were implanted into the muscular layer of the bowel with a needle as a trocar on the gastric antrum, the proximal duodenum 1-2 cm from pylorus; on the duodenum, jejunum, and ileum 1-2, 25-30, 95-100 cm distal to the pylorus, respectively in all groups. The bundled electrode wires were grasped by the clamp through a silastic tube, which then passed through the subcutaneous tunnel and then the abdominal wall was closed. Guinea pigs recovered from the surgery were similar to those of the controls. During this period, feces were collected daily to determine fecal bile acids.
Motility recordings One month after the cholecystectomy, the experiments were performed in conscious guinea pigs after being fasted for 16 h. The gastrointestinal myoelectric activity was continuously recorded by using an electrical swivel mechanism to a computerized, eight-channel recorder (RM-6280C, Chengdu, China) at least for 8 h for each animal, and during this period at least 2 or 3 MMCs appeared. Myoelectric activity was sampled at a rate of 1 kHz. The signals were amplified and bandpass filtered (frequencies above 0.3 Hz and below 100 Hz were cut off). After the conversion from analog to digital, the signals were stored on optical disk for later analysis.
MMC definition The different phases III of the MMC were defined as follow: Phase I = quiescence; Phase II = irregular contractions; and phase III = regular contractions with a duration of at least 2-3 min and migrating downwards in the gastrointestinal tract. The cycle length of the MMC was defined from the end of one phase III activity to the end of the following phase III activity.
An isotope dilution method for measurement of the bile acid pool size was described previously[3]. Each guinea pig of all groups was intraperitoneally injected [3H] Taurocholic acid 0.25 Ci. After the fasting 12 h period, guinea pigs were anesthetized under ketamine and the abdomen was opened. Bile was obtained via gallbladder or common bile duct puncture and amount of fasting bile was measured. The gallbladder bile was examined for cholesterol gallstones and for cholesterol crystals under regular and polarized light by light microscopy as previously described[4]. For storage, bile samples were mixed with an equal volume of ethanol and refrigerated at 4 °C.
For determination of bile acid concentration of radioactivity, an aliquot of bile-ethanol solution was extracted with equal volume of petroleum ether. The petroleum ether was discarded, and an aliquot of the ethanol phase was evaporated. Eleven microliters of scintillation liquid was added to 0.5 mL of bile sample in scintillation vial. After complete mixing, the mixture was stored at 4 °C overnight. The mixture was counted with a liquid scintillation counter (GJRT, Xi’an, China) and cpm value was obtained. (dpm = cpm/E%) Using external standardization to correct for quenching. The rest of bile-ethanol solution was used for analyzing concentration of total bile acids by capillary gas chromatography method as previously described[5].
Because the isotopic taurocholic acid is distributed randomly in the entire bile acid pool, total bile acid pool size can be calculated from the following formula:
PT = dpmA (BT/dpmT)
Where PT is the total bile acid pool, dpmA is the number of disintegrations per minute administered, BT is the concentration of total bile acids, and dpmT is the concentration of radioactivity.
Data are presented as mean±SD and analyzed by one-way analysis of variance (ANOVA) and t test. P values less than 0.05 were considered statistically significant. The SPSS 11.0 Statistical Soft-ware was used for statistical evaluations.
Fresh gallbladder bile samples from animals in normal diet group (A group and B group) were clear. There were no visible precipitates. In contrast, solid cholesterol crystals were observed in 27 of 30 animals in cholesterol fed animals (C group and D group) and macroscopic cholesterol gallstones were observed in the rest of animals.
Fasting gallbladder volume (FV), Residual volume (RV), Fasting gallbladder bile (FB), and Ejection fraction (E) were not different between A group and B group, C group and D group (Table 1). In contrast to above, FV, RV, and FB, were significantly increased in C group (P<0.05) and D group (P<0.05) compared with A group and B group, respectively whereas cholesterol feeding inhibited gallbladder empting, the ejection fraction falling 61.84% in C group (P<0.05); 61.23% in D group (P<0.05, Table 1).
After cholesterol feeding, bile acid pool size (PT) declined 57.23% (P<0.05) in C group and 55.24% (P<0.05) in D group. Bile acid in gallbladder bile (BA) was found in lower concentrations in C group (P<0.05) and D group (P<0.05) compared with control animals. Further, total fecal bile acid (FBA) outputs that reflect new bile acid synthesis were significantly raised 1.3 times (P<0.05) in B group, 3.3 times (P<0.05) in C group, 3.6 times (P<0.05) in D group compared with control group, respectively (Table 2). Although there were slight changes in PT, BA and FBA, these changes were not significant before and after surgery in normal and GS guinea pigs (Table 2).
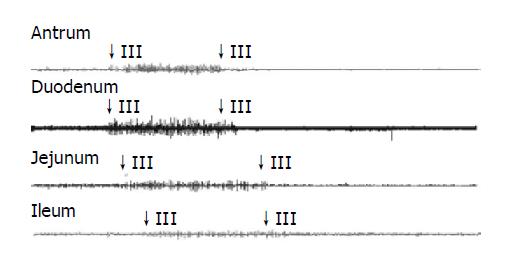
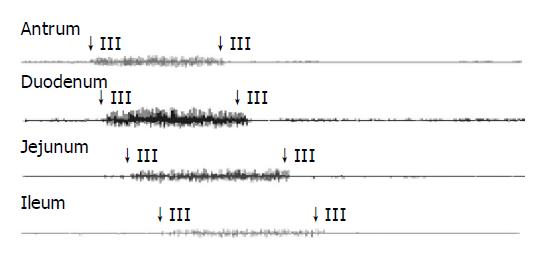
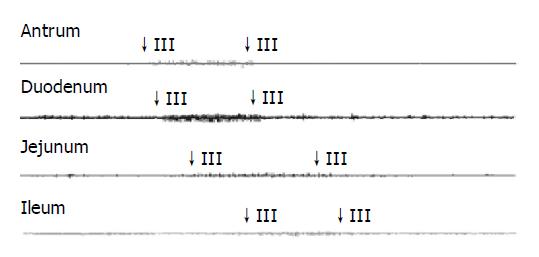
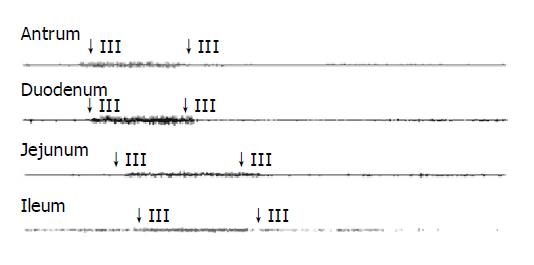
The typical pattern of MMCs was observed in all but one guinea pig, where no phase III activity was recorded. The total number of MMCs cycles recorded was 130. The mean MMC cycle duration in antrum (82.02±5.66 min), duodenum (83.07±5.59 min), jejunum (84.28±6.00 min), and ileum (90.41±7.39 min) in control group (Table 3). In total, phase III activities were observed with 46 (35%) of antral origin and 84 (65%) of duodenal origin and were propagated caudad in all animals.
| MMC (min) | A | B | C | D | |
| Antrum | Cycle duration | 82.02±5.66 | 80.25±5.20 | 114.19±7.82ac | 112.70±10.38ac |
| Phase I | 20.69±2.24 | 20.56±2.20 | 22.79±2.72 | 22.58±2.59 | |
| Phase II | 57.85±5.05 | 56.26±4.68 | 88.65±8.57ac | 87.44±8.64ac | |
| Phase III | 3.47±0.36 | 3.44±0.34 | 2.74±0.25ac | 2.67±0.25ac | |
| Duodenum | Cycle duration | 83.07±5.59 | 82.21±5.90 | 119.46±8.02ac | 118.05±10.55a |
| Phase I | 19.79±2.04 | 19.68±1.97 | 21.23±2.79 | 21.14±2.65 | |
| Phase II | 59.56±5.84 | 58.86±5.71 | 95.40±9.72a | 94.14±9.58 | |
| a Phase III | 3.73±0.39 | 3.66±0.37 | 2.83±0.31ac | 2.78±0.29ac | |
| Jejunum | |||||
| Cycle duration | 84.28±6.00 | 83.47±6.20 | 118.54±10.14ac | 117.36±9.49ac | |
| Phase I | 21.27±2.38 | 21.18±2.30 | 22.34±3.11 | 22.21±2.52 | |
| Phase II | 58.84±6.03 | 58.17±5.75 | 92.38±9.35ac | 91.41±9.03ac | |
| Phase III | 4.17±0.43 | 4.12±0.42 | 3.82±0.37ac | 3.75±0.36ac | |
| Ileum | Cycle duration | 90.41±7.39 | 88.37±7.01 | 434.00±21.57ac | 430.53±17.57ac |
| Phase I | 19.77±2.15 | 19.56±2.03 | 21.79±2.88ac | 21.60±2.71ac | |
| Phase II | 66.22±6.75 | 64.46±6.38 | 409.04±20.20ac | 405.81±18.29ac | |
| Phase III | 4.42±0.45 | 4.35±044 | 3.16±0.33ac | 3.12±0.31ac |
See in Figures 1, 2, 3, and 4. The results were expressed graphically to increase duodenum origin of MMC in C group (78.79%), in D group (80.95%), whereas 50% in control group. The prolonged MMC cycle length due to increased duration of phase II with lower contractile incidence from antrum to ileum in C group and D group. More pronounced fluctuations in duration of phase III appeared with significantly lower values in GS formation animals compared to normal animals. Furthermore, The frequency of phase III was remained unchanged whereas the amplitude was reduced by approximately 50% in all gastrointestinal recording sites in GS formation animals (Table 4). Slight parallel falls in duration of phase I, II, III, and MMC cycle duration but increase in amplitude, although not significant, were also seen in cholecystectomized guinea pigs compared to the intact guinea pigs either normal diet or cholesterol diet (Tables 3 and 4).
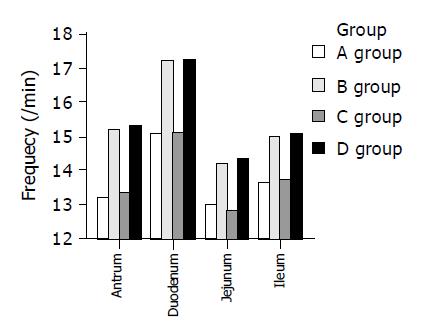
It was surgery not cholesterol diet that affected the MMC frequency in guinea pigs (Figure 5). The frequency of MMC was increased from 13.21±1.04 /min to 15.23±1.25 /min in antrum, 15.09±1.10 /min to 17.16±1.33 /min in duodenum, 12.97±1.19 /min to 14.21±1.12 /min in jejunum, and 13.65±1.03 /min to 14.98±1.20 /min in ileum (all P<0.05) in normal guinea pigs. Furthermore, postoperative frequency rose 15% (13.34±1.06 /min vs 15.30±1.19 /min, P<0.05) in antrum, 14% (15.13±1.13 /min vs 17.22±1.35 /min, P<0.05) in duodenum, 12% (12.86±1.14 /min vs 14.37±1.15 /min, P<0.05) in jejunum, and 10% (13.74±1.08 /min vs 15.07±1.24 /min in ileum, P<0.05) in GS guinea pigs. There was no significant difference in the other parameters of MMC before and after cholecystectomy.
In enterohepatic circulation of bile acids, bile acids are synthesized in the liver and then secreted into the biliary tree. They enter the lumen of the proximal small intestine. Ultimately, they reach the distal small intestine and are reabsorbed into the portal circulation to return to the liver, thus completing the cycle. Theoretically, both necessary steps of enterohepatic circulation-intestinal reabsorption and hepatic synthesis could influence the size of the bile acid pool.
Previous study indicated that an important factor influencing MMC was enterohepatic circulation of bile acids. The intact enterohepatic cycle is essential for initiation of regular duodenal MMC. Under normal conditions, Motilin, may be released as a consequence of the presence of bile in the duodenum via stimulating duodenal mucosa, induces phase III contractions of MMC[6]. During phase III activity, bile entered the duodenum in single drops, only in between two consecutive contractions, or as a series of drops during transient inhibition of duodenal contractions[7]. Therefore, contractions of duodenal wall result in bile system and junction of common bile duct and duodenum contractions, which preventing bile flow.
After cholecystectomy, bile loses storage and Oddi sphincter relatively relaxes, which result in bile acid flux continually through intestine and consequently an increase in the frequency of enterohepatic bile acid cycling. In this study, a statistically significant two-fold increase in fecal bile acids was also seen postoperatively. It implied short-term regulation of bile acid pool size by affecting increased fecal losses. Furthermore, 1 mo after removal of the gallbladder, we observed that bile acid concentration was not change because continuous transhepatic flux of bile acids probably resulted in feedback inhibition of fluid secretion[8]. As a result, we speculate that the entire small intestine is exposed to rapid flux but normal concentration of bile acids. Conceivably, no excess bile reaches and is incorporated into the smooth muscle membranes of small intestine. This modifies membrane fluidity and may not thereby affect function of the membrane regulator proteins, such as the receptors and ion channels that control electrical excitability and influence the periodicity of events like the MMC. This study shown that cycle length, duration of phase III, and amplitude of MMC were not significantly altered in removing gallbladder guinea pigs, which supported this hypothesis. Another possible explanation is that some receptors that have a threshold of bile acid concentrations may exist between the portal vein and the intrahepatic bile duct seems to be essential for a normal duodenal MMC cycle[9]. After surgery, stimulation threshold of bile acid is similar to those obtained from preoperative animals result from unchanged bile acid concentration in bile, which eventually do not alter MMC, as we observed.
In particular, it is thought that postcholecystectomy syndrome has been linked to the decrease of gastrointestinal motility[10]. In the contrary, this report has shown that characteristics of MMC were not affected by surgery in guinea pigs except for frequency of MMC. These results agree with Per Vadgaard Andersen’[11] clinical observation that cholecystectomy in patients with normal gallbladder function do not alter characteristics in duodenal motility. As a result, gastrointestinal dyskinesia is not involved in occurrence of postcholecystectomy syndrome.
We observed that bile acid pool sizes were coincident with changes of MMC in guinea pigs. Elimination of the gallbladder, bile acid pool size was enlarged while cycle length was shortened and amplitude was increased in MMC in normal guinea pigs. These changes were not significant. On the contrary, bile acid pool size was decreased approximately 50% in GS animals compared with normal animals, which was consistent with prolonged cycle length and decreased by 50% in amplitude of MMC in animal models that were under investigation. Furthermore, alterations of MMC and bile acid pool took place almost simultaneously. Therefore, no casual relationship could be demonstrated. One of the driving forces of the enterohepatic circulation is intestinal motility that provides the main propulsive force for bile acids to reach the site of intestinal absorption, and therefore has storage capacity for bile acid pool[12]. Thus, intestinal rather than hepatic alterations of EHC help maintain bile acid pool size. In the present study, bile acid pool size is maintained relatively constant at about 80-90 µmoL in control guinea pigs and 30-40 µmoL in GS guinea pigs by mechanism EHC after cholecystectomy. As a final note, we conclude that MMC might regulate bile acid pool through EHC in guinea pigs.
A further interesting observation was that after cholecystectomy, a increased, low amplitude, clustered contractions was seen during phase II of the interdigestive myoelectric complex in the ileum-analogous to the “discrete clustered contractions” (DCC)[13], but with shortened duration in three animals. These contractions propagated along terminal ileum during the fasted pattern of motility. It is likely that the occurrence of these waves were only in postoperative earlier stage occasionally because the recuperative time after the surgery was too short.
There was no information about preoperative gallbladder function in cholecystectomized patients in previous studies[10,11]. Therefore, guinea pigs with possible different preoperative gallbladder motility recordings were not excluded. The present study, however, no significant differences in gallbladder function were found before surgery in normal and GS guinea pigs, compensating this research defect. Most importantly, our study is the first to measure the bile acid pool sizes from preoperative and postoperative animals. It is therefore certain whether or not changes in the size of bile acid pool due to cholecystectomy.
In conclusion, we think that in cholecystectomized guinea pigs: (1) gastrointestinal motility is not affected by rapid frequency of cycling of the bile acid. (2) Intestinal bile acid flux is increased because bile acids secrete into the intestine is significantly increased. However, the former secondary to can suppress its own hepatic synthesis by increasing bile acid return through the liver in the end, bile acid kinetics reaching a new steady state. Thus, the bile acid pools eventually remain constant in guinea pigs. (3) There is a close relationship between the characteristics of MMC and bile acid pool size. These findings in this paper provide further stronger, but still indirect, evidence that gastrointestinal dyskinesia is not involved in occurrence of postcholecystectomy syndrome.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Gu S, Tang CW, Zhong XM. Investigation of postprandial phase of gallbladder emptying measured by ultrasonography. Chin J Gastroenterol. 2002;7:218-220. |
| 2. | Dodds WJ, Groh WJ, Darweesh RM, Lawson TL, Kishk SM, Kern MK. Sonographic measurement of gallbladder volume. AJR Am J Roentgenol. 1985;145:1009-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 189] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Chen L. Bile acid pool in the formation of pigment stone: an experimental study. Zhonghua WaiKe ZaZhi. 1992;30:496-48, 510. [PubMed] |
| 4. | Hayes KC, Stephan ZF, Pronczuk A, Lindsey S, Verdon C. Lactose protects against estrogen-induced pigment gallstones in hamsters fed nutritionally adequate purified diets. J Nutr. 1989;119:1726-1736. [PubMed] |
| 5. | Xu G, Salen G, Batta AK, Shefer S, Nguyen LB, Niemann W, Chen TS, Arora-Mirchandani R, Ness GC, Tint GS. Glycocholic acid and glycodeoxycholic acid but not glycoursocholic acid inhibit bile acid synthesis in the rabbit. Gastroenterology. 1992;102:1717-1723. [PubMed] |
| 6. | Stolk MF, van Erpecum KJ, Smout AJ, Akkermans LM, Jansen JB, Lamers CB, Peeters TL, vanBerge-Henegouwen GP. Motor cycles with phase III in antrum are associated with high motilin levels and prolonged gallbladder emptying. Am J Physiol. 1993;264:G596-G600. [PubMed] |
| 7. | Mochinaga N, Sarna SK, Condon RE, Dodds WJ, Matsumoto T. Gastroduodenal regulation of common duct bile flow in the dog. Gastroenterology. 1988;94:755-761. [PubMed] |
| 8. | Berr F, Stellaard F, Pratschke E, Paumgartner G. Effects of cholecystectomy on the kinetics of primary and secondary bile acids. J Clin Invest. 1989;83:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kajiyama Y, Irie M, Enjoji A, Ozeki K, Ura K, Kanematsu T. Role of bile acids in duodenal migrating motor complexes in dogs. Dig Dis Sci. 1998;43:2278-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Perdikis G, Wilson P, Hinder R, Redmond E, Wetscher G, Neary P, Adrian T, Quigley E. Altered antroduodenal motility after cholecystectomy. Am J Surg. 1994;168:609-14; discussion 614-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Andersen PV, Mortensen J, Oster-Jørgensen E, Rasmussen L, Pedersen SA, Qvist N. Cholecystectomy in patients with normal gallbladder function did not alter characteristics in duodenal motility which was not correlated to size of bile acid pool. Dig Dis Sci. 1999;44:2443-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Lanzini A, Lanzarotto F. Review article: the 'mechanical pumps' and the enterohepatic circulation of bile acids--defects in coeliac disease. Aliment Pharmacol Ther. 2000;14 Suppl 2:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Xu ED, Dong L, Lan K, Dai F, Song YH, Gong J, Luo JY. A study on antruo-duodenal motility in irritable bowel syndrome. Chin J Dig. 2003;23:142-145. |