Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3628
Revised: July 31, 2004
Accepted: March 10, 2005
Published online: June 21, 2005
AIM: To discuss the expression of human leukocyte antigen (HLA) class I antigens in gastric cancer and correlate these with pathologic type and TNM stage.
METHODS: The expression of HLA class I antigen was detected by immunohistochemistry in 185 specimens of gastric cancer, 20 gastric cancer specimens with lymphatic metastasis and 22 controls of normal gastric mucosa using four monoclonal antibodies.
RESULTS: The expression of HLA class I antigen (B/C locus) was significantly downregulated in gastric cancer and in lymphatic metastasis than that in normal gastric mucosa (χ2 = 7.712, P<0.05). The expression of other HLA class I antigens was also downregulated, but the change was slight. There was no relationship between the downregulation of HLA class I antigen and that of β2m and LMP2. The expression of HLA class I (B/C locus) was statistically correlated with pathologic stage in gastric adenocarcinoma (χ2 = 4.164, P<0.05).
CONCLUSION: The expression of HLA class I antigen (B/C locus) was obviously downregulated in gastric cancer and in lymphatic metastasis. This abnormal expression would provide the tumor cells with a way to avoid immunological recognition.
- Citation: Shen YQ, Zhang JQ, Miao FQ, Zhang JM, Jiang Q, Chen H, Shan XN, Xie W. Relationship between the downregulation of HLA class I antigen and clinicopathological significance in gastric cancer. World J Gastroenterol 2005; 11(23): 3628-3631
- URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3628
Gastric cancer is one of the most common forms of malignancy and is the major cause of mortality in human population of Jiangsu Province[1]. The development of gastric cancer is a multi-stage and multi-factor process and the host immune system plays an important role in defending tumor occurrence and metastasis. Human leukocyte antigen (HLA) is essential in anti-tumor immune response[2]. HLA molecules bind antigenic peptides generated by antigen processing machinery and present these peptides on the cell surface to T-cell receptor. The recognition of these peptides by CTLs triggers a series of events that can result in tumor cell lysis[3]. Downregulated expression of HLA class I molecules has been reported in many tumors of different origin including gastric cancer[4]. HLA class I antigen is a cell surface glycoprotein composed of heavy chain, β2m and a peptide. Any defect in the antigen processing progress such as the transporter proteins associated with antigen-processing TAP-1 and -2[5], the low molecular proteins LMP-2 and -7[6], and a TAP-associated protein Tapasin can result in the downregulation or loss of HLA class I antigen. In the present study, the expression of HLA class I antigen, β2m and LMP2 gene was detected in paraffin-embedded normal gastric mucosa, gastric cancer and lymphatic metastasis by the standard material and methods of international HLA work group[7]. The purpose was to explore the expression of HLA class I in gastric cancer and the relationship between the HLA class I expression and its clinical significance.
A total of 185 gastric cancer specimens (148 males and 37 females; age range 27-80 years), 22 normal gastric mucosa samples and 20 lymphatic metastasis samples were obtained from the Affiliated Hospital of Southeast University Medical School and the No. 1 Hospital of Lianyungang. All the samples were routinely fixed in 40 g/L formaldehyde solution, embedded in paraffin, and cut into 4-μm-thick sections. Samples were selected according to the pathologic diagnosis and reviewed by a pathologist to confirm the diagnosis.
Paraffin sections were deparaffinized with xylene and rehydrated by passage through decreased concentration of ethanol (from 100% to 70%). Endogenous peroxidase activity was blocked by a 20-min incubation at room temperature with 3% H2O2. Sections were then microwaved in citrate solution at 750 W for 10 min and preincubated with 2% normal horse serum for 30 min at room temperature followed by an overnight incubation at 4 °C with primary antibodies. Sections were incubated with biotinylated secondary antibody and then with ABC reagent at room temperature for 30 min. Diaminobenzidine solution was added to each section until desired stain intensity developed. Counterstain with hematoxylin, clear and mount. Primary antibodies HC-A2 (anti-HLA A locus; working dilution 1:100), HC-10 (anti-HLA B/C locus; working dilution 1:100), L368 (anti-β2m; working dilution 1:50) and SY-1 (anti-LMP2; working dilution 1:100) were a kind gift of Dr. Soldano Ferrone and Dr. Xin-Hui Wang (Department of Immunology, Roswell Part Cancer Institute, Buffalo, NY, USA).
The standard evaluation method was established at the 12th International Histocompatibility Conference[8]: The percentage and the intensity of stained cells were evaluated independently by at least two investigators: the percentage of stained cells in the whole section was scored as >75%, 25-75% and <25%; the staining intensity was scored as intense, weak and absent. The last scores combined by the precedent two factors as +,±, -. Lymphocyte and vascular endothelial cell present within the section were considered as positive control, while staining with the isotype matched irrelevant monoclonal antibody MK2-23 was used as negative control.
χ2 test was adopted to examine the relationship between the variables. A P value <0.05 was considered statistically significant.
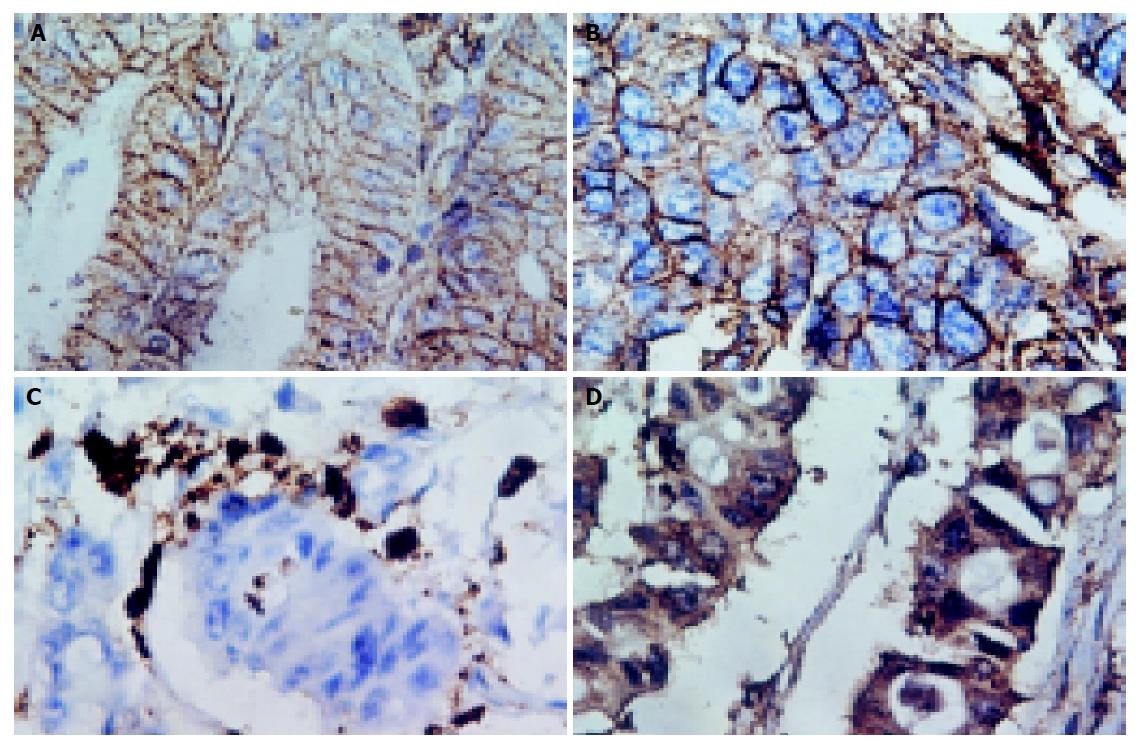
The staining pattern was same in normal gastric mucosas, gastric cancer, and lymphatic metastasis: the positive staining of HLA class I antigens was located in membrane, while the positive staining of β2m and LMP2 was located in cytoplasm and membrane (Figure 1).
Of the 22 normal gastric mucosa samples, 16 (73%) were classified as HLA class I (B/C locus) positive. There were 55 cases (35%) with positive HLA class I (B/C locus) expression in gastric cancer, while 5 cases (25%) in lymphatic metastasis. These results are shown in Table 1. The positive ratio was significantly higher in normal gastric mucosa than that in gastric cancer and in lymphatic metastasis (χ2 = 7.712, P<0.05). The expression of HLA class I (A locus), β2m and antigen processing molecular LMP2 was also downregulated, but the change was slight (data not shown). There was no relationship between the downregulation of HLA class I antigen and that of β2m and LMP2.
| Tissue | HLA class I antigen (B/C locus) expression | |||
| + | ± | - | Total | |
| Normal gastric mucosa | 16 (72.7) | 2 (9.1) | 4 (18.2) | 22 |
| Gastric cancer | ||||
| Histological grade | ||||
| I | 8 (42.1) | 6 (31.6) | 5 (26.3) | 19 |
| II | 13 (39.4) | 15 (45.5) | 5 (15.1) | 33 |
| III | 18 (26.1) | 23 (33.3) | 28 (40.6) | 69 |
| Other type | 16 (44.4) | 13 (36.1) | 7 (19.4) | 36 |
| Lymphatic metastasis | 5 (25) | 9 (45) | 6 (30) | 20 |
To further investigate the relationship between the expression of HLA class I antigen and the clinical pathology, we sorted the gastric carcinomas based on histological grades. Histological grade I means well-differentiated adenocarcinoma, II means moderately differentiated adenocarcinoma and III means poorly differentiated. The results indicated that expression of HLA class I (B/C locus) was statistically correlated with pathologic stage in gastric adenocarcinoma (χ2 = 4.164, P<0.05). We could not find any relationship between the expression of HLA class I antigen (B/C locus) and clinical TNM stage.
Recognition of tumor cells by cytolytic T lymphocytes depends on cell surface MHC class I expression. As a mechanism to evade T cell recognition, many malignant cancer cells, including gastric cancer, downregulate MHC class I. Ferron[9], Lopez-Nevot[10], Teh[11] have reported the expression of HLA antigen in gastric cancer in 1980s. But the results were not consistent because of different reagents and methods used by many laboratories. The “HLA expression in cancer” group established in the 12th International Histocompatibility Conference provided a series of standard reagents and methods to several labs that focus on HLA expression in cancer and its correlation with disease progression. Using the same criteria, the research teams could compare their data with others. At the International “HLA Expression in Cancer” reference laboratory, we investigated HLA molecule expression in gastric cancer, which is one of the most common forms of malignancy in Jiangsu Province by using the standard materials and methods of international HLA work group, and correlated these with pathologic type and TNM stage.
In this study, we first investigated the expression of HLA class I antigen in normal gastric mucosa, gastric cancer and lymphatic metastasis. The results indicated that HLA class I antigen (B/C locus) was lowly expressed in gastric cancer and in lymphatic metastasis compared with normal gastric mucosa, which was similar to the report from Klein[12]. HLA class I antigen is a cell surface glycoprotein composed of heavy chain, β2m and a peptide. Any defect in the antigen processing progress such as LMP2 can result in the downregulation or loss of HLA class I antigen. In gastric cancer, we found that the change of β2m and LMP2 were relatively slight and there was no statistical relationship between the downregulation of HLA class I antigen and that of β2m and LMP2. That is to say, other mechanisms may contribute to this downregulation. In our observation it was the change of HLA heavy chain at DNA and transcription level that lead to HLA class I antigen downregulation (to be published).
We also found that the downregulation of HLA class I antigen (B/C locus) was statistically correlated with pathologic stage in gastric adenocarcinoma. The data shown in Table 1 demonstrated that the expression of HLA class I antigen was higher in high-differentiated adenocarcinoma, while it decreases at advanced stage. The low-differentiated adenocarcinoma, which had lower expression of HLA class I antigen, may have more opportunity to escape from host immune surveillance. This may contribute to its rapid progression and poor prognosis.
In conclusion, the expression of HLA class I antigen (B/C locus) was obviously downregulated in gastric cancer and in lymphatic metastasis. This abnormal expression would provide the tumor cells with a way to avoid immunological recognition. Because recognition of tumor cells by cytolytic T lymphocytes depends on cell surface MHC class I expression, the downregulation might be an obstacle for T-cell-based immunotherapy or peptide vaccination that is of great interest at present[13,14]. For this reason, it is necessary to select the patients who positively express HLA antigen before clinical therapy[15] and it is important to find the mechanism underlying this abnormal expression and a way to promote it in gastric cancer.
| 1. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. 1990-1992 mortality of stomach cancer in China. Zhonghua ZhongLiu ZaZhi. 2002;24:4-8. [PubMed] |
| 2. | Finke J, Ferrone S, Frey A, Mufson A, Ochoa A. Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today. 1999;20:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Phan GQ, Wang E, Marincola FM. T-cell-directed cancer vaccines: mechanisms of immune escape and immune tolerance. Expert Opin Biol Ther. 2001;1:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Garrido F, Ruiz-Cabello F, Cabrera T, Pérez-Villar JJ, López-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 5. | Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Chang CC, Campoli M, Ferrone S. HLA class I defects in malignant lesions: what have we learned? Keio J Med. 2003;52:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 268] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Kurokohchi K, Carrington M, Mann DL, Simonis TB, Alexander-Miller MA, Feinstone SM, Akatsuka T, Berzofsky JA. Expression of HLA class I molecules and the transporter associated with antigen processing in hepatocellular carcinoma. Hepatology. 1996;23:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Ferron A, Perez-Ayala M, Concha A, Cabrera T, Redondo M, Oliva MR, Ruiz-Cabello F, Garrido F. MHC class I and II antigens on gastric carcinomas and autologous mucosa. J Immunogenet. 1989;16:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | López-Nevot MA, Esteban F, Ferrón A, Gutiérrez J, Oliva MR, Romero C, Huelin C, Ruiz-Cabello F, Garrido F. HLA class I gene expression on human primary tumours and autologous metastases: demonstration of selective losses of HLA antigens on colorectal, gastric and laryngeal carcinomas. Br J Cancer. 1989;59:221-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Teh M, Lee YS. HLA-DR antigen expression in intestinal-type and diffuse-type gastric carcinoma. Cancer. 1992;69:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Klein B, Klein T, Nyska A, Shapira J, Figer A, Schwartz A, Rakovsky E, Livni E, Lurie H. Expression of HLA class I and class II in gastric carcinoma in relation to pathologic stage. Tumour Biol. 1991;12:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Nie Y, Wu K, Yang J, Tian F, Li L, Chen B, Fan D. Induction of T lymphocytes specific to human gastric cancer using HLA-A matched allogeneic gastric tumor cells. J Immunother. 2003;26:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sato Y, Shomura H, Maeda Y, Mine T, Une Y, Akasaka Y, Kondo M, Takahashi S, Shinohara T, Katagiri K. Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre-existing cellular response to peptide. Cancer Sci. 2003;94:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394-3400. [PubMed] |