Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3574
Revised: May 1, 2004
Accepted: June 29, 2004
Published online: June 21, 2005
AIM: Dieulafoy’s lesion (DL) accounts for 1-5.8% of cases of acute upper gastrointestinal bleeding (GIB). Its mortality is high, approaching 20%, despite recent advances in endoscopic therapy. We aimed to report our experience in the treatment of DL.
METHODS: A retrospective case study of all patients with DL between January 1993 and January 2003 was done. Characteristics, treatment methods, success rates and 30-d mortality of the patients were analyzed.
RESULTS: Thirty-six patients were noted to have DL in the study period. Thirty-three records were available for assessment in which 35 DL were identified. The median age of the patients was 67 years with male to female ratio of 5.6:1. Significant comorbidities existed in 69% of the patients. Eighty-nine percent of the DL was found at first endoscopy, three DL at laparotomy. Significant coexistent endoscopic findings existed in 23%. Hemostasis was achieved in 88% by using adrenaline injection, or in combination with heater probe application at first endoscopy. Four cases had re-bleeding, all were successfully treated endoscopically. The 30-d mortality rate was 23%.
CONCLUSION: Successful endoscopic hemostasis could be achieved in 100% of cases of DL. The overall mortality may still remain high, mainly due to the comorbidities and age of these patients.
- Citation: Walmsley R, Lee YT, Sung JJ. Dieulafoy’s lesion: A case series study. World J Gastroenterol 2005; 11(23): 3574-3577
- URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3574.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3574
The rupture of a submucosal persistent-caliber artery, named after the famous 19th century French surgeon as Dieulafoy’s lesion (DL), is now a well-recognized cause of non-variceal upper gastrointestinal bleeding (GIB)[1,2]. Previously published case series have found DL to account for 1-5.8% of cases of acute non-variceal upper GIB[3-6]. In a prospective study, Matsui et al[7], found that 40% of all causes of upper GIB were due to DL rather than gastro-duodenal ulceration or varices.
DL is also a rare cause of bleeding from other parts of the gastrointestinal tract. The two largest retrospective series demonstrated that DL was the source of hemorrhage in 1.2-1.9% of all endoscopies performed for acute GIB[5,8].
By utilizing the range of endoscopic therapies available in a modern endoscopy department, hemostasis can be achieved in over 90% of cases[6,7,9-12], although several endoscopies may have to be performed for diagnostic and therapeutic purposes. The mortality, however, remains high approaching 20%, mainly because of the associated comorbidities encountered in these patients[4,5,8,9,13-16].
We aimed to review our experience in the treatment of DL and to compare the results with published data.
A retrospective review of the GIB registry from January 1993 to the end of January 2003 was undertaken in the Prince of Wales Hospital, a teaching hospital that serves a catchment population of around 1.2 million. The GIB registry is a prospective registry of all patients admitted for GIB who undergo endoscopic examinations. All procedures were carried out by a group of experienced therapeutic endoscopists. The agreed endoscopic criteria for the diagnosis of DL were the presence of active arterial spurting or visualization of a protruding vessel from a minute mucosal defect that had no obvious underlying ulceration[17]. The case notes of patients were retrieved and reviewed. Clinical data including presenting symptoms, current medication, comorbidities, hemoglobin and hematocrit at the time of GIB, and number of units of blood transfused were assessed. Endoscopic findings of the appearance and site of DL, total number of endoscopies and endoscopic therapies undertaken were noted along with the need for surgical intervention, 30-d rebleeding and mortality rate. Re-bleeding was considered to have occurred if the hemoglobin dropped by 2 g/dL, or the patients became shocked with further evidence of GI blood loss after initial blood transfusion and stabilization, or if there was endoscopically confirmed re-bleeding from the DL on repeated endoscopy.
During the study period, 15100 patients were admitted due to GIB. The source of bleeding in 1288 patients was variceal in origin. Thirty-six patients were diagnosed to have DL, accounting for 0.26% of all non-variceal GIB during this period. One patient was noted at initial endoscopy to have bleeding from a DL, but the lesion was considered actually to be an esophageal varix with stigmata of recent hemorrhage at repeated endoscopy on the next day. The patient was therefore excluded from this analysis. The hospital records of two patients could not be retrieved. Thirty-three case records were analyzed.
The mean follow-up time was 373 d (range 3-2340 d). The patient demographics and clinical data are given in Table 1.
| Age: median (range) (yr) | 67 (33-92) |
| M:F | 28:5 |
| Concomitant medications used | 24 |
| NSAIDs or aspirin | 8 |
| Anticoagulation | 3 |
| Comorbidities | |
| Sepsis | 8 |
| Advanced malignancy | 4 |
| Cardiovascular disease | 5 |
| Renal failure | 3 |
| Chronic obstructive pulmonary disease | 4 |
| Alcoholism | 3 |
| Prior Billroth II gastrectomy | 4 |
| Hemoglobin at presentation: median (range) (g/dL) | 7.4 (4.2-15.7) |
| Hematocrit at presentation: median (range) | 20% (13-46) |
| Units of blood transfused: median (range) | 4 (0-15) |
There were 35 DL in the 33 patients. Twenty patients presented with melena alone, six with hematemasis, five with both hematemasis and melena and two with frank hematochezia. Four patients were hemodynamically compromised at presentation, three were clinically shocked and one had postural hypotension.
Only nine patients were not receiving any medication at the time of presentation. Two patients were on warfarin treatment: one for atrial fibrillation complicated by brachial artery embolus, the other for a previous mechanical aortic valve replacement. One diabetic patient was on prophylactic low molecular weight heparin as he was immobile due to cellulitis and gout.
In addition to four cases of advanced malignancies at presentation, two other patients had asymptomatic carcinoma of the prostate, one underwent surgical treatment for bronchial carcinoma 10 years ago, and the second received a mastectomy for carcinoma of the breast 12 years prior to presenting with GIB.
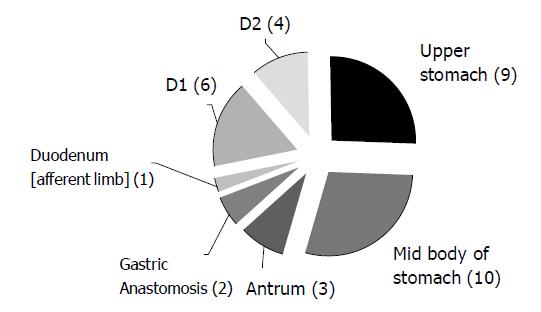
The anatomical locations of DL are shown in Figure 1.
All were found by esophago-gastro-duodenoscopy (EGD). No colonic DL was reported during the study period. Two patients had two DLs, one in the afferent loop of a gastro-duodenostomy and also at the duodenal papilla, and the other had DLs on the lesser curve of the stomach as well as at the pylorus.
The endoscopic appearance of DL at the time of diagnostic EGD included active spurting (n = 22), adherent clot (n = 7) and visible vessel alone (n = 5). One report contained no detailed description of the lesion. In a 68-year-old gentleman with melena and 6.8 g/dL of hemoglobin, the DL was seen only as a vague protuberant scar and was subsequently confirmed by the demonstration of submucosal vessels in that area by an endoscopic ultrasound (EUS) examination.
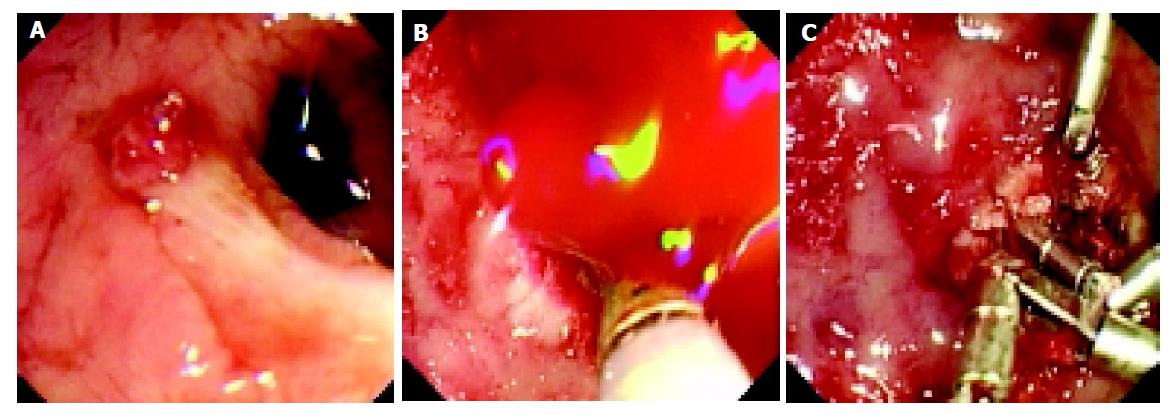
The majority of DLs (86%, 30/35) were diagnosed in the first EGD examination, only two patients were diagnosed at a second endoscopy and the remaining three DLs were found at laparotomy. A mean of 1.4 EGD sessions (range 1-3) was needed for diagnosing and/or treating the DLs. Adrenaline (1:10000) injection plus heat probe therapy was the mainstay of therapy used (n = 30). The aim of the heat probe therapy was set to completely abolish the visible DL. Other therapies employed included adrenaline injection alone (n = 2), heat probe therapy alone (n = 2) and hemoclipping (n = 2). In one patient, hemoclipping was successfully used when heat probe therapy failed to control the bleeding (Figure 2). There were no complications of endoscopic intervention.
Coexistent significant endoscopic findings were common. They consisted of one gastric ulcer, one duodenal ulcer, significant gastric erosions in two, severe duodenitis in three and a small column of esophageal varices in one. Among the 16 patients tested, 7 (44%) were found to have Helicobacter pylori infection. Adjuvant acid suppressive therapy was given in all patients in the form of H2 blocker (n = 10), proton pump inhibitor (n = 22), or intravenous proton pump inhibitor followed by H2 blocker in one patient.
Hemostasis was achieved at the first EGD in 88% (29/33). Four patients had re-bleeding after initial hemostasis, three within 24 h and one at 72 h. All achieved second hemostasis by repeated endoscopic therapy.
In three patients the DL were only revealed during laparotomy for uncontrolled upper GIB after the initial endoscopy. One patient had successful treatment of a lesser curve DL but continued to bleed and was found to have a pyloric channel spurting DL at surgery that was dealt with by undersewing the lesion. In a second case the primary endoscopy found a bleeding gastric ulcer, which was treated endoscopically, but continued blood loss necessitated a laparotomy that revealed a posterior fundal DL. A wedge resection was undertaken. In a third case no bleeding source could be found at EGD and a posterior gastric wall DL was only unveiled by gastrostomy. Again a wedge resection of this area of the stomach was performed.
The median time of hospital stay post presentation with GIB was 4 d (range 1-28 d). The 30 d mortality was 27% (nine patients) with GIB contributing directly to the death in three patients. The median age at the time of death was 75 years (range 41-92 years).
This is the third largest case series of DL in comparison to the published series[5,8]. Our patients were of a similar age range to that described in previous studies and the presence of significant comorbidities in the majority of patients (69%) at the time of presentation was also close to that in other reports[4,5,8,9,11]. However, there was a marked male to female preponderance of 5.6:1, twice that generally found in the literature[4-6,9,10,15].
Some studies have found a significant proportion of GIB patients with DL to be on NSAIDs or aspirin and it has been suggested that erosive gastritis and subsequent vessel wall necrosis induced by these compounds might precipitate the rupture of submucosal persistent caliber vessels[4,5,11,19]. Our findings that only 24% of our study patients were taking NSAID or aspirin at the time of presentation do not support this hypothesis.
The finding of significant concurrent GI pathologies in 7 (21%) cases is not uncommon. In one case series 11% of 90 patients with DL had peptic ulcers while 18% had significant erosions[5]. In another report, 43% of patients had ‘significant’ endoscopic pathologies[8].
The exact sites of DL are in line with the recognized distribution. The ‘classic’ position for DL is on the high lesser curve, within 6 cm of the cardia[5,18]. In our series 41% of the 22 DLs found in stomachs with normal anatomy were in the classical position. It is interesting that we found a number of DLs in patients with previous Billroth II gastrectomies (10%). One DL was located in the afferent limb of the duodenum and in the other two cases the bleeding point was situated along the anastomotic margin. This co-existence of DL with prior gastric surgery was also shown by Nikolaidis et al[11], who found 57% of 23 patients had prior gastric surgery and 43% of the DLs in these cases were located at the anastomotic site[11]. The location of DLs at the anastomotic site did not affect therapeutic treatment outcome.
The initial hemostasis rate of endoscopic treatment was nearly 90% and the overall success rate was 100%, which were similar to recent published data[6,7,11,12]. Rebleeding after initially successful hemostasis was recognized as being relatively common, reported to be in the range of 6-28%[3,9,13,16,17]. During the 10 years of the study, the predominant method of treatment was a combination of local 1:10000 adrenaline injection followed by heater probe application. There is a tendency to use mechanical methods for hemostasis, such as hemoclips[9,10], and endoscopic banding ligation (EBL)[7,10,11,12,20]. These methods were reported to give a successful hemostasis rate of 75-100%. From the limited data available it appears that EBL may have a slightly lower rebleeding rate than combined injection and coagulation treatment[7,12,21].
We used EUS to confirm the diagnosis of DL in one patient. The resolution available, aided by color Doppler, allowed detection of the large caliber (2-3 mm) vessels penetrating the muscularis mucosa and has been used by a number of groups to direct and assess the success of ablative treatments[22-25].
The 30-d mortality rate in our series was 27% and most of the patients died due to serious coexisting medical diseases rather than GIB. The case series published, since the widespread use of endoscopic methods for hemostasis, has been a common place report with a similar overall death rate[3,8,15,16]. The advanced median age of patients who died within 30 d of their presentation with GIB is in keeping with the findings of other studies that age and comorbidities are powerful predictive factors of a poor outcome in GIB[26].
In conclusion DL is a rare condition with a high overall mortality rate. Our series of consecutive DL patients presenting over the past 10 years reflects the success of endoscopic therapy for this intriguing disorder. A combination of injection and heat probe therapy can achieve a remarkable hemostasis rate so that surgery can be avoided.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Mikó TL, Thomázy VA. The caliber persistent artery of the stomach: a unifying approach to gastric aneurysm, Dieulafoy's lesion, and submucosal arterial malformation. Hum Pathol. 1988;19:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Dieulafoy G. Exulceratio simplex. Clin méd de l’ Hôtel-Dieu de Paris 1897/98, II; L’intervention chirurgicale dans les hématémèses foudroyantes consécutives á l’exulceration simple de l’estomac [French] Pr méd. 1898;29-44. |
| 3. | Pointner R, Schwab G, Königsrainer A, Dietze O. Endoscopic treatment of Dieulafoy's disease. Gastroenterology. 1988;94:563-566. [PubMed] |
| 4. | Baettig B, Haecki W, Lammer F, Jost R. Dieulafoy's disease: endoscopic treatment and follow up. Gut. 1993;34:1418-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Norton ID, Petersen BT, Sorbi D, Balm RK, Alexander GL, Gostout CJ. Management and long-term prognosis of Dieulafoy lesion. Gastrointest Endosc. 1999;50:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Kasapidis P, Georgopoulos P, Delis V, Balatsos V, Konstantinidis A, Skandalis N. Endoscopic management and long-term follow-up of Dieulafoy's lesions in the upper GI tract. Gastrointest Endosc. 2002;55:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Matsui S, Kamisako T, Kudo M, Inoue R. Endoscopic band ligation for control of nonvariceal upper GI hemorrhage: comparison with bipolar electrocoagulation. Gastrointest Endosc. 2002;55:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Schmulewitz N, Baillie J. Dieulafoy lesions: a review of 6 years of experience at a tertiary referral center. Am J Gastroenterol. 2001;96:1688-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Parra-Blanco A, Takahashi H, Méndez Jerez PV, Kojima T, Aksoz K, Kirihara K, Palmerín J, Takekuma Y, Fuijta R. Endoscopic management of Dieulafoy lesions of the stomach: a case study of 26 patients. Endoscopy. 1997;29:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Chung IK, Kim EJ, Lee MS, Kim HS, Park SH, Lee MH, Kim SJ, Cho MS. Bleeding Dieulafoy's lesions and the choice of endoscopic method: comparing the hemostatic efficacy of mechanical and injection methods. Gastrointest Endosc. 2000;52:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Nikolaidis N, Zezos P, Giouleme O, Budas K, Marakis G, Paroutoglou G, Eugenidis N. Endoscopic band ligation of Dieulafoy-like lesions in the upper gastrointestinal tract. Endoscopy. 2001;33:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Mumtaz R, Shaukat M, Ramirez FC. Outcomes of endoscopic treatment of gastroduodenal Dieulafoy's lesion with rubber band ligation and thermal/injection therapy. J Clin Gastroenterol. 2003;36:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Reilly HF, al-Kawas FH. Dieulafoy's lesion. Diagnosis and management. Dig Dis Sci. 1991;36:1702-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Stark ME, Gostout CJ, Balm RK. Clinical features and endoscopic management of Dieulafoy's disease. Gastrointest Endosc. 1992;38:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Katz PO, Salas L. Less frequent causes of upper gastrointestinal bleeding. Gastroenterol Clin North Am. 1993;22:875-889. [PubMed] |
| 16. | Skok P. Endoscopic hemostasis in exulceratio simplex-Dieulafoy's disease hemorrhage: a review of 25 cases. Endoscopy. 1998;30:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Dy NM, Gostout CJ, Balm RK. Bleeding from the endoscopically-identified Dieulafoy lesion of the proximal small intestine and colon. Am J Gastroenterol. 1995;90:108-111. [PubMed] |
| 18. | Veldhuyzen van Zanten SJ, Bartelsman JF, Schipper ME, Tytgat GN. Recurrent massive haematemesis from Dieulafoy vascular malformations--a review of 101 cases. Gut. 1986;27:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Juler GL, Labitzke HG, Lamb R, Allen R. The pathogenesis of Dieulafoy's gastric erosion. Am J Gastroenterol. 1984;79:195-200. [PubMed] |
| 20. | Wong RM, Ota S, Katoh A, Yamauchi A, Arai K, Kaneko K, Yazawa M, Matsuzaki F. Endoscopic ligation for non-esophageal variceal upper gastrointestinal hemorrhage. Endoscopy. 1998;30:774-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Abi-Hanna D, Williams SJ, Gillespie PE, Bourke MJ. Endoscopic band ligation for non-variceal non-ulcer gastrointestinal hemorrhage. Gastrointest Endosc. 1998;48:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Fockens P, Meenan J, van Dullemen HM, Bolwerk CJ, Tytgat GN. Dieulafoy's disease: endosonographic detection and endosonography-guided treatment. Gastrointest Endosc. 1996;44:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Folvik G, Nesje LB, Berstad A, Odegaard S. Endosonography-guided endoscopic band ligation of Dieulafoy's malformation: a case report. Endoscopy. 2001;33:636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Nesje LB, Skarstein A, Matre K, Myking AO, Odegaard S. Dieulafoy's vascular malformation: role of endoscopic ultrasonography in therapeutic decision-making. Scand J Gastroenterol. 1998;33:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Ribeiro A, Vazquez-Sequeiros E, Wiersema MJ. Doppler EUS-guided treatment of gastric Dieulafoy's lesion. Gastrointest Endosc. 2001;53:807-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Vreeburg EM, Terwee CB, Snel P, Rauws EA, Bartelsman JF, Meulen JH, Tytgat GN. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |