Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3319
Revised: April 5, 2004
Accepted: May 29, 2004
Published online: June 7, 2005
AIM: To investigate the mRNA expression of gamma-aminobutyric acid A (GABAA) receptor subunits α1, β1, γ2 in different parts of the brain of rats with hepatic encephalopathy.
METHODS: Twelve adult male Sprague-Dawley rats were randomly divided into two groups: (1) hepatic encephalopathy model group (n = 6), which was induced by intraperitoneal injection of thioacetamide (TAA, 350 mg/kg) for three consecutive days; (2) control group (n = 6), in which the rats were treated with same dose of normal saline solution. After the freeze slice of cerebrum was made, in situ hybridization was used to detect the mRNA of GABAA receptor subunits α1, β1, and γ2 in rat cerebral cortex, basal nuclei, substantia nigra and hippocampi. Image data were collected and analyzed quantitatively by QWin550CW model image signal gather and analysis system.
RESULTS: In rats with hepatic encephalopathy, mRNA expression levels of GABAA receptor subunits α1, β1 increased significantly in basal nuclei, substantia nigra pars compacta, substantia nigra pars reticularis and hippocampi (144.7±15.67/184.14±4.41, 60.61±33.66/113.07±32.44, 87.71± 21.25/128.40±18.85, 122.34±5.56/161.60±4.56, 123.29±5.21/140.65±4.15, 123.40±4.42/140.09±4.52, 124.76±4.18/140.09±4.12, 141.62±15.09/182.80 ±5.20, 69.13±30.74/134.21±43.76, 87.87±25.16/151.01±19.49, 122.14±6.30 /162.33±3.92, 122.81±5.09/137.19±7.12, 123.00±4.63/138.11±5.92, 125.75 ±2.43/138.81±6.10, P<0.01), but did not change in the cerebral cortex compared to the control group. Similar changes were found in the mRNA expression levels of GABAA receptor subunit γ2, which increased significantly in basal nuclei, substantia nigra pars compacta, substantia nigra pars reticularis (136.81±26.41/167.97±16.23, 51.00±36.14/113.18±36.52, 86.35±20.30/ 126.90±19.74, P<0.01), CA1 of hippocampal (162.15±9.05/178.62±6.45, P<0.05), and no changes were found in the cerebral cortex and CA2, CA3, CA4 of hippocampi.
CONCLUSION: In rats with hepatic encephalopathy, mRNA expression levels of GABAA receptor subunits α1, β1, γ2 increase significantly in basal nuclei, substantia nigra and hippocampi, suggesting that the changes of mRNA expression levels in GABAA receptor subunits may contribute to the pathogenesis of hepatic encephalopathy.
- Citation: Li XQ, Dong L, Liu ZH, Luo JY. Expression of gamma-aminobutyric acid A receptor subunits α1, β1, γ2 mRNA in rats with hepatic encephalopathy. World J Gastroenterol 2005; 11(21): 3319-3322
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3319.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3319
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome associated with fulminant hepatic failure and chronic liver disease. Its pathogenesis is unclear till now. One of the important factors is the increase of gamma-aminobutyric acid (GABA) level and GABA-ergic activity. GABA is the most important inhibitory neurotransmitter in the central nervous system (CNS). Up to now, three different GABA receptors have been discovered: GABAA, GABAB and GABAC receptors. Among these three receptors, GABAA receptor is considered to have the closest relationship with HE. In order to find out the pathogenesis of HE, we used in situ hybridization to detect the changes of mRNA expression of GABAA receptor subunits α1, β1, and γ2 in several major parts of the brain of rats after making hepatic encephalopathy models by intraperitoneal injection of thioacetamide.
Twelve adult male Sprague-Dawley rats (230±17 g), supplied by Xi’an Jiaotong University Experiment Animal Centre were used in this study. The rats were randomly divided into hepatic encephalopathy model group (n = 6) which was induced by intraperitoneal injection of thioacetamide (TAA, 350 mg/kg) for three consecutive days, and control group (n = 6) in which the rats were treated with same dose of normal saline solution.
Rats were poured with 40 g/L polymerisatum/0.1 mol/L PBS with 1 mL/L DEPC for fixation after being anesthetized. Cerebrum was taken out, fixed with 40 g/L polymerisatum/0.1 mol/L PBS containing 1 mL/L DEPC for 20 min, and soaked in 300 g/L cane sugar solution. Before bein cut into 14-μm thick slices, the slides were dealt with poly-L-lysine.
Digoxin-labeled oligonucleotide probes aimed directly at GABAA receptor subunits α1, β1, γ2 were used for in situ hybridization (ISH). Experiments were carried out according to ISH detection kit manufacturer’s instructions (Boster Company, Wuhan). Slices were dealt with 1:50 mixed 30 mL/L H2O2 and pure methanol for 30 min at room temperature. After washing thrice with distilled water, the slices were digested with fresh pepsin that was diluted in 3% citromalic acid for 5-120 s at room temperature in order to expose mRNA nucleic acid fragment, then washed thrice (5 min each time) with PBS for ISH, followed by a wash with distilled water. After washing, the slices were fixed with 40 g/L polymerisatum/0.1 mol/L PBS containing 1 mL/L DEPC for 10 min at room temperature, and subsequently washed thrice with distilled water. After all the preparatory procedures were completed, we added 20 μL of prehybridization liquid to each slice and prehybridized it for 2-4 h at 38-42 °C. Then the unnecessary liquid was removed without washing. Thereafter, we added 20 μL of hybridization liquid to each slice and covered it with a cover slip. After hybridizing overnight at 38-42 °C, the slices were washed twice (5 min each time) with 2×SSC at 37 °C, once for 15 min with 0.5×SSC at 37 °C, 1-3 times (15 min each time) with 0.2×SSC at 37 °C, and finally treated with close liquid for 30 min at 37 °C. Unnecessary liquid was removed without washing before biotin-conjugated rat anti-digoxin antibody was added to the slices. After reaction for 60 min at 37 °C, the slices were washed four times (5 min each time) with PBS for ISH. Then, we added streptavidin-biotin complex (SABC). After reaction for 20 min at 37 °C, the slices were washed thrice (5 min each time) with PBS for ISH. Then biotin-conjugated peroxidase was added to react for 20 min at 37 °C, washed four times (5 min each time) with PBS for ISH. After the color was developed by DAB, the slices were washed sufficiently with water. At last, all slices were dehydrated with alcohol, and sealed.
Ten ISH slices were randomly selected from each brain region in both groups. Image data were collected and analyzed quantitatively using QWin550CW model image signal gather and analysis system.
Data analysis was done with SPSS10.0 statistics software. The average gray values were compared between control and hepatic encephalopathy model group with t test. P<0.05 was considered statistically significant.
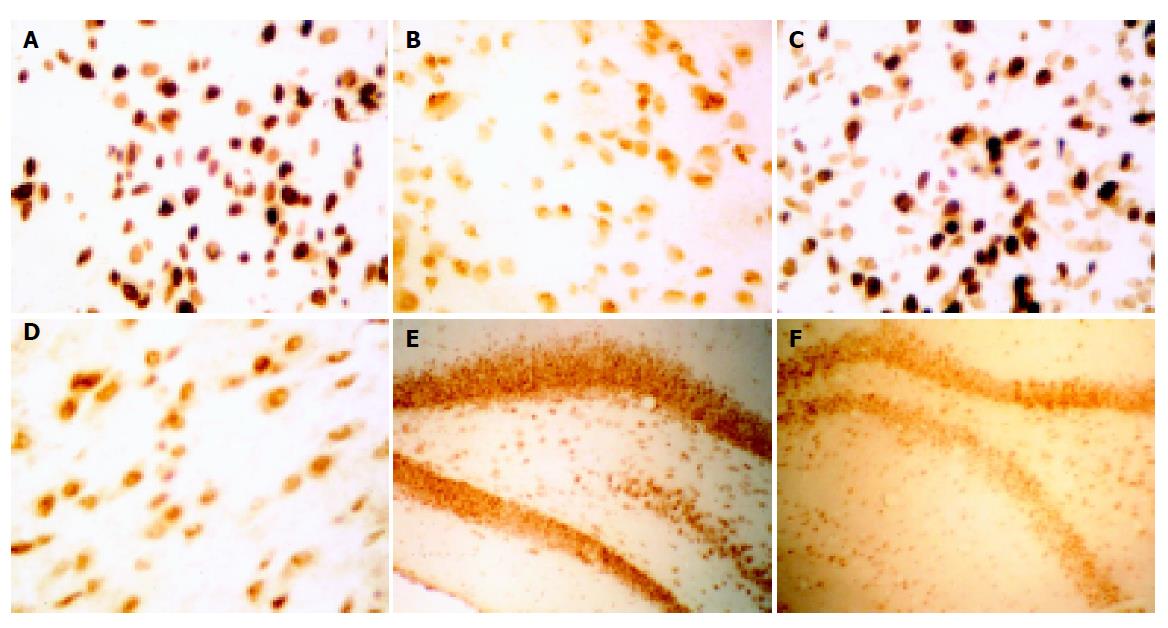
The positively stained GABAA receptor subunits α1, β1, γ2 mRNA were located in cytoplasm of the cells. Table 1 shows the results of quantitative analysis of mRNA expression levels of GABAA receptor subunits α1, β1, γ2 in rats with hepatic encephalopathy using QWin550CW model image signal gather and analysis system.
| Position | α1 mRNA | β1 mRNA | γ2 mRNA | |||
| Control group | HE model | Control group | HE model | Control group | HE model | |
| Basal nuclei | 184.14±4.41 | 144.7±15.67b | 182.80±5.20 | 141.62±15.09b | 167.97±16.23 | 136.81±26.41b |
| Cerebral cortex | 149.54±13.74 | 154.9±11.02 | 141.96±17.55 | 155.26±10.24 | 142.47±15.69 | 143.29±24.87 |
| Substantia nigra pars reticularis | 113.07±32.44 | 60.61±33.66b | 134.21±43.76 | 69.13±30.74b | 113.18±36.52 | 51.00±36.14b |
| Substantia nigra pars compacta | 128.40±18.85 | 87.71±21.25b | 151.01±19.49 | 87.87±25.16b | 126.90±19.74 | 86.35±20.30b |
| Hippocampal CA1 | 161.60±4.56 | 122.34±5.56b | 162.33±3.92 | 122.14±6.30b | 178.62±6.45 | 162.15±9.05a |
| Hippocampal CA2 | 140.65±4.15 | 123.29±5.21b | 137.19±7.12 | 122.81±5.09b | 162.70±5.90 | 161.57±6.40 |
| Hippocampal CA3 | 140.09±4.52 | 123.40±4.42b | 138.11±5.92 | 123.00±4.63b | 162.09±5.35 | 161.36±5.04 |
| Hippocampal CA4 | 140.09±4.12 | 124.76±4.18b | 138.81±6.10 | 125.75±2.43b | 158.97±6.12 | 160.1±5.21 |
In rats with hepatic encephalopathy, mRNA expression levels of GABAA receptor subunits α1, β1 increased significantly in basal nuclei, substantia nigra pars compacta, substantia nigra pars reticularis and hippocampi (P<0.01), but did not change in the cerebral cortex compared to the control group. Similar changes were found in the mRNA expression levels of GABAA receptor subunit γ2, which increased significantly in basal nuclei, substantia nigra pars compacta, substantia nigra pars reticularis (P<0.01), CA1 of hippocampi (P<0.05), and no changes were found in the cerebral cortex and CA2, CA3, CA4 of hippocampi. (Figure 1, Table 1)
Although many research works were reported in recent years that tried to explain the pathogenesis of hepatic encephalopathy, it is still not elucidated properly[1,2]. According to the increased levels of GABA in plasma, increased permeability of the blood-brain barrier and the changes of GABA receptors on the surface of neuron membrane, Schafer and other scientists proposed the GABA hypothesis about hepatic encephalopathy in 1982[3,4]. GABA is a principal inhibitory neurotransmitter in the mammalian brain, and the concentration of GABA in plasma is increased in the animal model of liver function failure during hepatic encephalopathy. The increased concentration of GABA neurotransmitter and enhanced GABA-ergic activity are considered to be associated with the development of hepatic encephalopathy in the central nervous system[5-7].
Up to now, 3 GABA receptors GABAA, GABAB and GABAC have been reported, and GABAA receptor has been extensively studied in recent years. GABAA receptor, a chloride-permeable channel, can increase permeability for chlorions when activated and thereafter mediate inhibitory postsynaptic potentials (IPSPs), thus inducing inhibitory effects. Benzodiazepine (BDZ) can enhance these intermediary effects directly or indirectly. Moreover, the function of GABA receptors can also be mediated by alcohol, evanescent narcotic and steroid after binding to the sites on these receptors[8,9]. GABA receptor is an oligomer, which consists of seven subunits, α1-6, β1-4, γ1-4, δ, ρ1-3, ε, and Π, and each subunit has a different function. Now it is deemed that β subunit is the binding site for GABA and GABAA receptors, and α and γ subunits of GABAA can react with BDZ[10,11]. Among these subunits, α1, β2, and γ2 account for 43% of all the subunits, and γ2 subunit presents in 60% of GABAA receptors. The pharmacological analysis proved that BDZ has a much more enhancing effect on the receptors that consisted of α1, β3, and γ2 subunits than those consisted of α2, α5, α6, and γ2 subunits[12]. The presence of γ2 subunit can up-regulate BDZ[13].
In this study, the rat model of hepatic encephalopathy induced by thioacetamide was chosen to study the mRNA expression level of α1, β1, and γ2 subunits of GABAA receptors on the different regions of brain by ISH. The results showed that the mRNA expression level of α1, β1, and γ2 subunits significantly increased in the areas of basal nuclei, substantia nigra pars compacta and pars reticularis, suggesting that hepatic encephalopathy is related with the diversity of mRNA level expression of GABAA receptor subunits. When the mRNA expression level of β1 significantly increased, the number of binding sites for GABA and GABAA receptors increased, the GABA-ergic tension was enhanced, the number of open chlorion tunnels was increased, and inhibitory neurotransmitter effects were enhanced. The mRNA expression level of α1 and γ2 subunits significantly increased, the sensitivity of GABAA receptors for BDZ was enhanced, and BDZ could induce conformational change of receptors, the affinity of GABA for its receptors was increased, leading to enhancement of the GABA-ergic tension. According to these findings, we could explain the reason why hepatic encephalopathy can be effectively treated with the specific antagonist of Benzodiazepine (Flumazenil)[14,15].
GABA is the most important inhibitory transmitter of substantia nigra-corpus striatum pathway and by-pass[16]. Most neurons in corpus striatum are multi-dendritic spine GABA-ergic neurons (75%), and these neurons may be the unique source of efferent nerve fibers of corpus striatum (send fibers to pallidum and substantia nigra). GABA can be found in all globus pallidus, and has a concern with the efferent nerve fibers of pallidum-substantia nigra pars reticularis. Substantia nigra is divided into the dorsal pars compacta and ventral pars reticularis, and most neurons in pars reticularis are GABA-ergic neurons. The ventral part of substanti nigra can extend to thalamus. Furthermore, it is presumed to connect with pallidum and has a similar structure with it[17]. The GABA-ergic fibers from lateral globus pallidus reach both pars compacta and pars reticularis, and most fibers terminate at pars reticularis. The projection pathways of GABA-ergic neurons of pars reticularis are as follows: (1) The fibers reach nucleus ventralis anterior thalami and mediodorsal nucleus through nigrothalamic tract, then project to the prefrontal and cingulum cortex after relay; (2) The fibers reach pontine nucleus and reticular formation through pathway, then the impulse transmits to anterior column neurons of spinal cord after relay; (3) The fibers from substantia nigra-tectum tract project at homolateral superior colliculus, and reach reticular formation of medulla oblongata and spinal cord. Nucleus ventralis anterior thalami regulates the motion, and participates in ascending activation. Mediodorsal nucleus participates in superordinary action of cortex, and has a concern with emotion. In our study, we found that the expression of α1, β1 and γ2 subunits significantly increased in basal nuclei of Meynert, substantia nigra pars compacta and pars reticularis, that is to say, GABA-ergic tension of substantia nigra-corpus striatum pathway and by-pass was enhanced, which may be the molecular mechanism for the multi-psychiatric and neuropathic symptoms during hepatic encephalopathy.
Hippocampal formation has a concern with emotion, learning and memory, sleep and wakefulness, etc. In our study, we also found that the expression of α1 and β1 subunits significantly increased in all areas of hippocampus during hepatic encephalopathy, and the expression level of γ2 subunit increased in the CA1 region, suggesting that GABA-ergic tension is enhanced in those regions, and this is correlated with the symptoms of motion change, and sleep reverse during hepatic encephalopathy.
In summary, our results show that in rats with hepatic encephalopathy, mRNA expression levels of GABAA receptor subunits α1, β1, γ2 increase significantly in basal nuclei, substantia nigra and hippocampi, suggesting that the changes of GABAA receptor subunits mRNA expression levels may contribute to the pathogenesis of hepatic encephalopathy. At present, the pathogenesis of hepatic encephalopathy is studied at the transcriptional level of GABAA receptor subunits. The expression abnormality of other subunits of receptors is also involved during hepatic encephalopathy, meanwhile, the expression of receptors is regulated by post-translational subunits, and also influenced by receptor assembly, transportation, insertion on the membrane. All these require further research.
| 1. | Chu CJ, Lee FY, Wang SS, Chang FY, Lin HC, Wu SL, Chan CC, Tsai YT, Lee SD. Establishment of an animal model of hepatic encephalopathy. Zhonghua YiXue ZaZhi (Taipei). 2000;63:263-269. [PubMed] |
| 2. | Jones EA. Ammonia, the GABA neurotransmitter system, and hepatic encephalopathy. Metab Brain Dis. 2002;17:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Schafer DF, Jones EA. Hepatic encephalopathy and the gamma-aminobutyric-acid neurotransmitter system. Lancet. 1982;1:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 189] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Schafer DF, Jones EA. Potential neural mechanisms in the pathogenesis of hepatic encephalopathy. Prog Liver Dis. 1982;7:615-627. [PubMed] |
| 5. | Jones EA. Pathogenesis of hepatic encephalopathy. Clin Liver Dis. 2000;4:467-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Wysmyk U, Oja SS, Saransaari P, Albrecht J. Enhanced GABA release in cerebral cortical slices derived from rats with thioacetamide-induced hepatic encephalopathy. Neurochem Res. 1992;17:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Zhang Z, Wu C, Zhou P. Changes in gamma-amino-butyric acid and N-methyl-D-aspartic acid receptor-gated currents from freshly isolated hippocampal CA1 pyramidal neurons of hepatic encephalopathy rats. Zhonghua YiXue ZaZhi. 1997;77:439-442. [PubMed] |
| 8. | Akk G, Steinbach JH. Low doses of ethanol and a neuroactive steroid positively interact to modulate rat GABA(A) receptor function. J Physiol. 2003;546:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ahboucha S, Desjardins P, Chatauret N, Pomier-Layrargues G, Butterworth RF. Normal coupling of brain benzodiazepine and neurosteroid modulatory sites on the GABA-A receptor complex in human hepatic encephalopathy. Neurochem Int. 2003;43:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | 1998 Receptor and ion channel nomenclature. Trends Pharmacol Sci. 1998;Suppl:1-98. [PubMed] |
| 11. | Crestani F, Assandri R, Täuber M, Martin JR, Rudolph U. Contribution of the alpha1-GABA(A) receptor subtype to the pharmacological actions of benzodiazepine site inverse agonists. Neuropharmacology. 2002;43:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Costa E, Guidotti A. Benzodiazepines on trial: a research strategy for their rehabilitation. Trends Pharmacol Sci. 1996;17:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798. [PubMed] |
| 14. | Dursun M, Caliskan M, Canoruc F, Aluclu U, Canoruc N, Tuzcu A, Yilmaz S, Isikdogan A, Ertem M. The efficacy of flumazenil in subclinical to mild hepatic encephalopathic ambulatory patients. A prospective, randomised, double-blind, placebo-controlled study. Swiss Med Wkly. 2003;133:118-123. [PubMed] |
| 15. | Ferenci P, Grimm G. Benzodiazepine antagonist in the treatment of human hepatic encephalopathy. Adv Exp Med Biol. 1990;272:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Farrant M, Webster RA. Compartmental distribution of endogenous amino acids in the substantia nigra of the rat. Brain Res. 1989;480:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Webster KE. Structure and function of the basal ganglia - a non-clinical view. Proc R Soc Med. 1975;68:203-210. [PubMed] |