Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3099
Revised: October 20, 2004
Accepted: January 5, 2005
Published online: May 28, 2005
AIM: Model of End-stage Liver Disease (MELD) score has recently gained wide acceptance over the old Child-Pugh score in predicting survival in patients with decompensated cirrhosis, although it is more sophisticated. We compared the predictive values of MELD, Child-Pugh and creatinine-modified Child-Pugh scores in decompensated cirrhosis.
METHODS: A cohort of 102 patients with decompensated cirrhosis followed-up for a median of 6 mo was studied. Two types of modified Child-Pugh scores estimated by adding 0-4 points to the original score using creatinine levels as a sixth categorical variable were evaluated.
RESULTS: The areas under the receiver operating charac-teristic curves did not differ significantly among the four scores, but none had excellent diagnostic accuracy (areas: 0.71-0.79). Child-Pugh score appeared to be the worst, while the accuracy of MELD was almost identical with that of modified Child-Pugh in predicting short-term and slightly better in predicting medium-term survival. In Cox regression analysis, all four scores were significantly associated with survival, while MELD and creatinine-modified Child-Pugh scores had better predictive values (c-statistics: 0.73 and 0.69-0.70) than Child-Pugh score (c-statistics: 0.65). Adjustment for gamma-glutamate transpeptidase levels increased the predictive values of all systems (c-statistics: 0.77-0.81). Analysis of the expected and observed survival curves in patients subgroups according to their prognosis showed that all models fit the data reasonably well with MELD probably discriminating better the subgroups with worse prognosis.
CONCLUSION: MELD compared to the old Child-Pugh and particularly to creatinine-modified Child-Pugh scores does not appear to offer a clear advantage in predicting survival in patients with decompensated cirrhosis in daily clinical practice.
-
Citation: Papatheodoridis GV, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V, Archimandritis AJ. MELD
vs Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol 2005; 11(20): 3099-3104 - URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3099
The poor survival of patients with decompensated cirrhosis has driven physicians to a constant search for good prognostic markers[1,2]. The need for improvement in the accuracy of prognosis in this setting has increased in the current era of the expansion of orthotopic liver transplantation (OLT) and the parallel increasing discrepancy between the numbers of OLT candidates and the numbers of available donor livers[2,3]. Indeed, patients with decompensated cirrhosis who are potential OLT candidates should enter into the transplant waiting lists neither too early nor too late, since early OLTs take away livers from candidates who are very sick and with more urgent indications and late OLTs are associated with worse outcomes[4].
The old Child-Turcotte classification[5] and the subsequently modified Child-Pugh score (CP score)[6] have been the most widely applied prognostic markers in patients with decom-pensated cirrhosis mainly due to their simplicity for use in daily clinical practice[2,7,8]. The determination of CP score, which may range from 5 to 15, is based on the presence and severity of ascites and hepatic encephalopathy, the prolongation of prothrombin time, and the levels of serum bilirubin and albumin. According to their CP scores, patients are classified into three classes (Child class A, B, and C with CP scores 5-6, 7-9, and 10-15, respectively)[6]. The predictive value of the CP score has been shown in many studies in the past[2,9], but the inclusion of two subjective variables (ascites and enceph-alopathy) with the inevitable interobserver variation and the need for even better prognosis have prompted the search for more objective and more accurate prognostic markers in this setting[2].
During the last two decades, several scoring systems or prognostic instruments have been proposed for predicting survival in patients with decompensated cirrhosis[10-13], but none gained wide acceptance until the recent development of the Mayo Clinic Model of End-stage Liver Disease (MELD)[2,14,15]. MELD score[16], which was initially developed for predicting survival in patients undergoing transjugular intrahepatic portosystemic shunts (TIPS)[17], has been suggested to provide more accurate prognosis than CP score in patients with decompensated cirrhosis and therefore to improve the estimation of priority for liver grafts allocation[2,16]. MELD score calculation is based on the etiology of cirrhosis and three simple and objective laboratory variables, serum bilirubin, serum creatinine and prothrombin time expressed as international normalized ratio (INR), but it includes logarithmic transfor-mations and multiplication by several factors being substantially more sophisticated than that of the CP score[6,17]. Moreover, it has been suggested that it may be difficult to reconcile clinical impression with MELD score[8]. Recently, a modified CP score taking into account serum creatinine levels was also evaluated in patients undergoing TIPS[18], since renal function in patients with decompensated cirrhosis has been shown to affect post-TIPS or post-transplant survival[19-21] and no renal parameter was included in the original CP score.
The aim of this study was to compare the accuracy of MELD, CP, and modified CP score for predicting short-term and medium-term survival in patients with decompensated cirrhosis.
We retrospectively studied 102 patients with decompensated cirrhosis, who were admitted to our department between June 1998 and May 2000. Patients with hepatocellular carcinoma, severe primary cardiopulmonary failure or intrinsic kidney disease were excluded, while patients with more than one admission during the study period were evaluated in the analysis only at their first admission.
The diagnosis of decompensated cirrhosis was based on clinical, laboratory, previous histological, and radiological signs of cirrhosis with at least one sign of liver decompensation (ascites, variceal bleeding, hepatic encephalopathy, non-obstructive jaundice). The cause of cirrhosis was considered to be chronic hepatitis B virus infection in cases with long-standing (>6 mo) HBsAg positivity; chronic hepatitis C virus (HCV) infection in cases with detectable both antibodies against HCV (anti-HCV) and serum HCV-RNA; alcohol abuse in cases with a compatible history and absence of other causes of liver injury; primary biliary cirrhosis in cases with elevated alkaline phosphatase, positive antimitochondrial antibodies and/or compatible previous histological findings; primary sclerosing cholangitis in cases with elevated alkaline phosphatase and compatible radiological and/or histological findings.
According to our routine clinical practice, detailed medical history, complete physical examination, and a battery of laboratory tests were performed in all patients with decompensated cirrhosis on the day of admission. Moreover, diagnostic paracentesis and ascitic fluid culture were performed in all admitted cirrhotic patients with ascites. The age, sex, cause of cirrhosis, cause of admission, first and previous complications of decompensated cirrhosis including spontaneous bacterial peritonitis (SBP) as well as complete blood count including platelet count, prothrombin time and INR, serum urea and creatinine, total, and direct bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase, gamma-glutamate transpeptidase (GGT), serum albumin and globulins and ascitic fluid charac-teristics and culture were retrospectively recorded for all patients. In June 2002, the date of last available information as well as the final status (alive, death from liver disease, OLT, and death from liver unrelated causes) were recorded.
Based on the admission data, the CP score (range: 5-15) and Child class were estimated for each patient according to the suggestion by Pugh et al[6], while the MELD score (range: 6-40) was calculated according to the formula proposed by Kamath et al[16], which was a slight modification of the risk score used in the original TIPS model[17]. In addition, two types of modified CP score (CP score-I and CP score-II) with serum creatinine as a sixth variable were also calculated: CP score-I (range: 5-19) derived from the original CP score by adding 0 points for creatinine <1.3 mg/dL and 4 points for creatinine ≥1.3 mg/dL according to what was reported by Angermayr et al[18], while CP score-II (range: 5-19) derived from the original CP score by adding 0 points for creatinine <1.3 mg/dL, 2 points for creatinine 1.3-1.8 mg/dL and 4 points for creatinine >1.8 mg/dL.
All data were analyzed using the statistical program STATA. Results were expressed as mean values (SD) or as median values (range). Qualitative variables were compared by corrected χ2 test and quantitative variables by t-test or Wilcoxon signed rank test. The accuracy of the different score systems for predicting short-term survival was evaluated through the urea under the receiver operating characteristic (ROC) curve, whereas the different areas were compared by the non-parametric method proposed by DeLong et al[22].
Cox proportional hazards models were used to determine variables associated with overall survival. Multivariate models were constructed to identify independent variables from the score systems factors. For each Cox model, a predictive score was calculated for each patient as: P = β1X1+β2X2+…+βkXk, where X1, X2,…, Xk are the levels of k prognostic factors and β1, β2,…, βk are the corresponding regression coefficients. Higher predictive scores correspond to poorer prognosis. The accuracy of the different models as predictors of survival was evaluated by the concordance (c)-statistics (equivalent to the area under the ROC curve). Each model was considered to have diagnostic accuracy in case of a c-statistics >0.70 and excellent diagnostic accuracy in case of a c-statistics >0.80.
To assess how well the models fit the data predicted by the models and actual survival curves were graphically compared. For that, patients were ranked according to their predictive score and divided into three groups with roughly equal numbers of patients’ deaths in each group. Actual survival curve for each group was calculated using the Kaplan-Meier method.
The patient baseline characteristics are presented in Table 1. During a median follow-up of 16 mo (0.5-42 mo), 19 (19%) of the 102 patients died and another 5 (5%) underwent OLT. No patient died from liver unrelated causes. The 3-, 6-, 12-, and 24-mo survival rates were 91%, 86%, 84%, and 76%, respectively.
| Age (yr) | 61 (27-89) |
| Sex, males | 69 (68%) |
| Cause of cirrhosis | |
| HBV | 24 (23%) |
| HCV | 17 (17%) |
| Alcohol | 39 (38%) |
| PBC/PSC | 7 (7%) |
| Unknown | 15 (15%) |
| Cause of admission | |
| Tense ascites | 37 (36%) |
| Encephalopathy | 17 (17%) |
| Variceal bleeding | 16 (16%) |
| Jaundice | 5 (5%) |
| Fever | 11 (11%) |
| Other | 16 (16%) |
| SBP on admission | 9 (9%) |
| First sign of decompensation | |
| Ascites | 66 (65%) |
| Variceal bleeding | 30 (29%) |
| Encephalopathy | 5 (5%) |
| Jaundice | 1 (1%) |
| Hematocrit (%) | 33 (17-48) |
| Hemoglobin (g/dL) | 11 (5-16) |
| White blood count (×109/L) | 5.7 (1.2-23.9) |
| Platelet count (×109/L) | 105 (19-394) |
| Prothrombin time (s) | 16 (11-35) |
| INR | 1.3 (0.9-3.3) |
| Creatinine (mg/dL) | 1.1 (0.5-3.7) |
| Bilirubin (mg/dL) | 2.6 (0.3-33.9) |
| AST (IU/L) | 72 (20-610) |
| ALT (IU/L) | 44 (11-433) |
| Alkaline phosphatase (U/L) | 101 (42-487) |
| GGT (U/L) | 70 (10-1 165) |
| Albumin (g/dL) | 3.2 (1.9-4.3) |
| Child class | |
| A | 13 (13%) |
| B | 42 (41%) |
| C | 47 (46%) |
| Child-Pugh score | 9 (5-15) |
| Modified Child-Pugh score-I | 10 (5-19) |
| Modified Child-Pugh score-II | 10 (5-17) |
| MELD score | 12 (-10-45) |
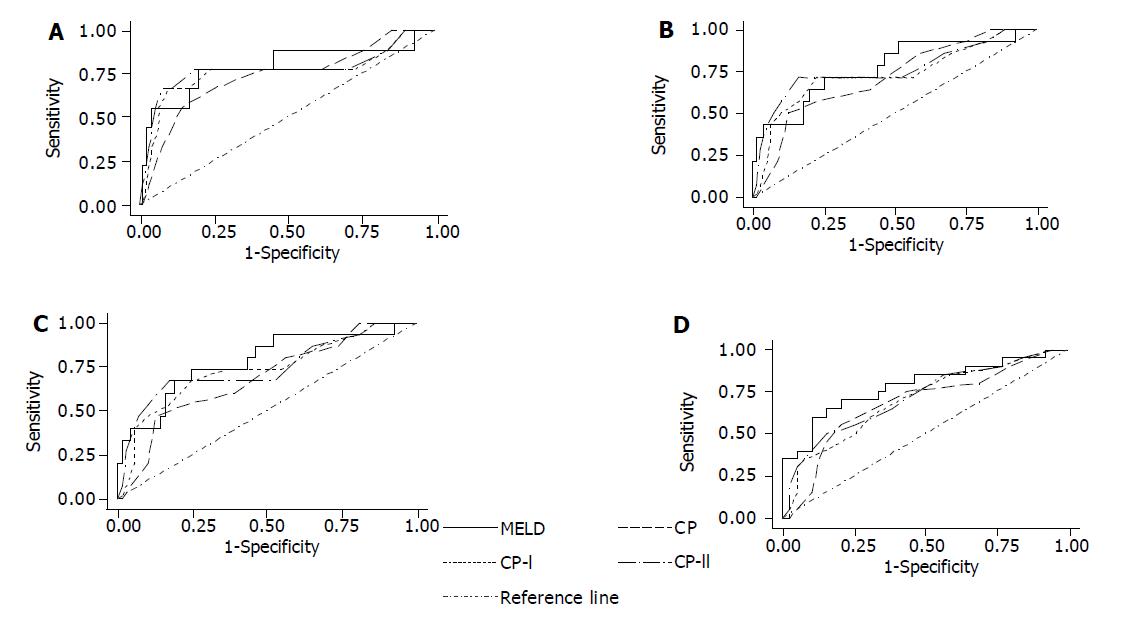
The ROC curves of all four scoring systems for 3-, 6-, 12-, and 24-mo survival are shown in Figure 1. Comparison of the areas under the ROC curves among the four scoring systems did not reveal any statistical significant difference. All scoring systems were found to have diagnostic accuracy in predicting survival, perhaps with the marginal exception of CP score for predicting 12-mo survival. However, none of the scores had excellent diagnostic accuracy (area >0.80). MELD score appeared to have better predictive accuracy compared to the CP scores. Among the rest of the scores, modified CP-I was associated with better area under the ROC curve than the CP score, whereas modified CP-II score was relatively more accurate in predicting short-term survival than the modified CP-I score. The area under the ROC curve for MELD and CP-II scores were almost identical for 3-mo (0.79 and 0.78) and 6-mo (0.77 and 0.76) survival, while there was a slight decrease in the predictive value of CP-II score during further follow-up. Thus, MELD appear to have a slight advantage in predicting 12- and 24-mo survival (0.78 and 0.79) compared with CP-II score (0.74 and 0.76), which ranked second (Table 2). In general, there was a tendency for decreasing accuracy of the CP scoring systems for predicting longer survival.
| Survival (mo) | Predictive scores | Area under ROC | P (χ2) |
| 3 | Child-Pugh | 0.73 | 0.19 |
| Modified Child-Pugh-I | 0.76 | 0.58 | |
| Modified Child-Pugh-II | 0.78 | 0.73 | |
| MELD | 0.79 | ||
| 6 | Child-Pugh | 0.71 | 0.18 |
| Modified Child-Pugh-I | 0.73 | 0.49 | |
| Modified Child-Pugh-II | 0.76 | 0.85 | |
| MELD | 0.77 | ||
| 12 | Child-Pugh | 0.68 | 0.09 |
| Modified Child-Pugh-I | 0.73 | 0.38 | |
| Modified Child-Pugh-II | 0.74 | 0.51 | |
| MELD | 0.78 | ||
| 24 | Child-Pugh | 0.7 | 0.27 |
| Modified Child-Pugh-I | 0.75 | 0.48 | |
| Modified Child-Pugh-II | 0.76 | 0.64 | |
| MELD | 0.79 |
Univariate analysis using Cox proportional hazards models showed that ascites as first sign of liver decompensation (P = 0.017), presence of SBP on admission (P = 0.010), serum levels of bilirubin (P<0.001), AST (P = 0.018), ALT (P = 0.039), GGT (P = 0.019), and creatinine (P = 0.002) were significantly associated with survival (Table 3).
| Patient characteristic | Hazard ratio | 95%CI | P |
| Age (per yr) | 0.99 | 0.90-1.03 | 0.72 |
| Sex (male) | 0.68 | 0.27-1.72 | 0.92 |
| Cause of cirrhosis (unknown) | |||
| HBV | 0.41 | 0.12-1.58 | 0.21 |
| HCV | 1.21 | 0.35-4.17 | 0.77 |
| Alcohol | 0.72 | 0.27-1.93 | 0.51 |
| PBC/PSC | 0.97 | 0.47-3.85 | 0.68 |
| SBP on admission | 4.25 | 1.41-12.84 | 0.01 |
| White blood count (×109/L) | 1.12 | 0.43-3.68 | 0.90 |
| Platelet count (×109/L) | 1.06 | 0.52-2.15 | 0.87 |
| Prothrombin time (s) | 2.48 | 0.50-12.19 | 0.26 |
| INR | 4.22 | 0.98-18.19 | 0.052 |
| Creatinine (mg/dL) | 4.62 | 1.76-12.13 | 0.012 |
| Bilirubin (mg/dL) | 2.25 | 1.5-3.38 | <0.001 |
| AST (IU/L) | 1.95 | 1.12-3.4 | 0.018 |
| ALT (IU/L) | 1.70 | 1.03-2.8 | 0.039 |
| Alkaline phosphatase (U/L) | 0.83 | 0.38-1.86 | 0.66 |
| GGT (U/L) | 1.64 | 1.09-2.46 | 0.019 |
| Albumin (g/dL) | 0.53 | 0.07-3.84 | 0.53 |
| Child class (A) | |||
| B | 2.11 | 0.46-9.77 | 0.34 |
| C | 3.71 | 0.78-17.61 | 0.1 |
MELD, CP, and modified CP-I and CP-II scores were all significantly associated with survival in univariate analysis. Multivariate Cox regression analysis including all significant baseline characteristics together with each predictive score showed that the only factor that remained consistently statistically significant for all scores was GGT (log-transformed). Table 4 shows the crude and the adjusted for GGT results for all scoring systems as well as the corresponding c-statistics. MELD and modified CP-II scores were again found to have the better predictive value for overall survival than CP score. Including GGT in the model increased substantially the value of the c-statistics.
| Scoring system | Unadjusted | Adjusted | ||
| HR (95%CI) | c-statisticsHR | (95%CI) | c-statistics | |
| Child-Pugh score | 1.23 (1.04-1.45) | 0.65 | 1.38 (1.14-1.68) | 0.77 |
| Modified Child-Pugh-I score | 1.21 (1.09-1.36) | 0.69 | 1.30 (1.13-1.50) | 0.79 |
| Modified Child-Pugh-II score | 1.30 (1.13-1.48) | 0.70 | 1.38 (1.18-1.62) | 0.78 |
| MELD score | 1.11 (1.06-1.15) | 0.73 | 1.10 (1.06-1.15) | 0.81 |
The expected and observed survival curves for each score in the three patient subgroups divided according to the patient respective predictive degree P are shown in Figure 2. The models fit the data reasonably well. MELD score appeared to discriminate better than the rest of the scores the subgroup of patients with the worse prognosis had.
Although the relatively new MELD score has already been instituted by some transplant programs, such as UNOS, to be the score of choice for stratification of liver transplant candidates on the waiting lists for allocation of donor livers[23], its superiority over the old CP score in predicting actual survival in patients with decompensated cirrhosis has not been documented. MELD score has been proven to be a reliable measure of short-term mortality risk in patients with end-stage liver disease and a suitable marker for allocation of donor livers[16,24], but the results in studies comparing MELD with CP score appear to be unclear. For prediction of survival in patients undergoing TIPS, MELD compared to CP score was found to be superior in the original MELD model study[17] and in a study from Italy[25], slightly superior in a subsequent study from Germany[26] and equally accurate in a study from Austria[19]. In patients with liver cirrhosis, however, both MELD and CP scores have been found to represent equally good predictors of survival without significant differences in the accuracy of their predictive values in all but one study population. Finally, MELD was found to be equivalent to CP score for predicting in-hospital and 12-mo mortality in patients with acute variceal bleeding.
Our data further support that MELD score is not significantly superior to CP score in predicting survival in patients with decompensated liver disease. The c-statistics for prediction of 3-, 6-, 12-, and 24-mo survival by the MELD score ranged from 0.75 to 0.79 being compatible with previous findings in other retrospectively evaluated cohorts of patients with decompensated cirrhosis[16]. Although no significant difference between MELD and CP score was observed in our study, the predictive accuracy of MELD score was always superior offering the greatest benefit in the prediction of 12- and 24-mo survival. The modified CP-I score with the addition of serum creatinine levels as a dichotomous categorical variable (0 points for creatinine <1.3 mg/dL and 4 points for creatinine ≥1.3 mg/dL) according to what was recently proposed by Angermayr et al[18], did not offer a clear benefit over the old CP score (c-statistics: 0.68-0.77 and 0.68-0.73, respectively). In contrast, the modified CP-II score with the addition of serum creatinine levels as a trichotomous categorical variable (0 points for creatinine <1.3 mg/dL, 2 points for creatinine = 1.3-1.8 mg/dL and 4 points for creatinine >1.8 mg/dL) was found to be slightly superior than the CP score, since its c-statistics (0.71-0.78) were always better than those of the CP score and very close to the c-statistics of the MELD score.
If two or more scoring systems offer similar accuracy in predicting survival, then other characteristics should be taken into account for adopting one of them into clinical practice. The main drawbacks of the old CP score are the inclusion of two subjective parameters, such as ascites and encephalopathy, and the estimation of three objective parameters, prothrombin time, serum bilirubin and albumin levels, as categorical variables[6]. Thus, in the CP score, there may be significant interobserver variation in the assessment of the severity of ascites and encephalopathy, which may easily change by medical interventions, while an extended range of values of prothrombin time, bilirubin and albumin levels take the same points even if they may reflect different degrees of liver failure. Moreover, CP score does not take into account the patient’s renal function, which appears to be strongly associated with survival[21].
MELD score is undoubtedly more objective than the CP score, since its calculation is based on the etiology of cirrhosis and three simple and reproducible laboratory parameters, INR, serum creatinine and bilirubin levels[16]. Moreover, the dynamic nature of MELD score, which is expressed within a continuous scale of 34 points taking into account the exact value of its laboratory parameters, offers an advantage in the determination of priorities of liver organs allocation[2,8]. On the other hand, MELD score cannot be calculated at the bedside and is much more complex than the easy to calculate CP score, since it includes logarithmic transformations and multiplication by several factors[16]. In addition, changes in several parameters of the MELD score may not be directly related to changes of the severity of liver disease, such as a creatinine increase due to extensive use of diuretics or other iatrogenic factors. Finally, it has been suggested that MELD score may underestimate the severity of liver disease in patients with decompensated cirrhosis and predominant complications of portal hypertension, since it does not include any parameter related to portal hypertension[8].
In conclusion, both MELD and CP score can accurately predict short-term (3- and 6-mo) survival in patients with decompensated cirrhosis, while MELD appears to have a slight advantage in predicting medium-term (12- and 24-mo) survival. The modified CP score with the addition of serum creatinine as a categorical parameter was found to improve the predictive accuracy of CP score being equivalent with the MELD score in predicting short- and medium-term survival. Thus, the creatinine-modified Child-Pugh scores seem to deserve further evaluation, since they are simpler than and of similar predictive accuracy with the MELD score and have higher predictive accuracy than the old Child-Pugh score. Taking into consideration the drawbacks of the MELD and CP scoring systems, we do not see a clear advantage in daily clinical practice for MELD score over the familiar and more easily calculated CP score or creatinine-modified CP score for predicting survival in patients with decompensated cirrhosis. Perhaps, the situation is different in the liver transplant waiting lists, since the dynamic range of the MELD score may allow better determination of priorities for organ allocation.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 358] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Forman LM, Lucey MR. Predicting the prognosis of chronic liver disease: an evolution from child to MELD. Mayo End-stage Liver Disease. Hepatology. 2001;33:473-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Lucey MR, Brown KA, Everson GT, Fung JJ, Gish R, Keeffe EB, Kneteman NM, Lake JR, Martin P, McDiarmid SV. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 266] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Freeman RB, Edwards EB. Liver transplant waiting time does not correlate with waiting list mortality: implications for liver allocation policy. Liver Transpl. 2000;6:543-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Child CG3, Turcotte JG. Surgery and portal hypertension. The Liver and Portal Hypertension. Philadelphia: Saunders 1964; 1-85. [Cited in This Article: ] |
| 6. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5490] [Cited by in F6Publishing: 5499] [Article Influence: 107.8] [Reference Citation Analysis (1)] |
| 7. | Conn HO. A peek at the Child-Turcotte classification. Hepatology. 1981;1:673-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 178] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Reuben A. Child comes of age. Hepatology. 2002;35:244-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 204] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Schlichting P, Christensen E, Andersen PK, Fauerholdt L, Juhl E, Poulsen H, Tygstrup N. Prognostic factors in cirrhosis identified by Cox's regression model. Hepatology. 1983;3:889-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 129] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Villeneuve JP, Infante-Rivard C, Ampelas M, Pomier-Layrargues G, Huet PM, Marleau D. Prognostic value of the aminopyrine breath test in cirrhotic patients. Hepatology. 1986;6:928-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Adler M, Verset D, Bouhdid H, Bourgeois N, Gulbis B, Le Moine O, Van de Stadt J, Gelin M, Thiry P. Prognostic evaluation of patients with parenchymal cirrhosis. Proposal of a new simple score. J Hepatol. 1997;26:642-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Cooper GS, Bellamy P, Dawson NV, Desbiens N, Fulkerson WJ, Goldman L, Quinn LM, Speroff T, Landefeld CS. A prognostic model for patients with end-stage liver disease. Gastroenterology. 1997;113:1278-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 679] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | McCaughan GW, Strasser SI. To MELD or not to MELD? Hepatology. 2001;34:215-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3434] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 17. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 1911] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 18. | Angermayr B, Koenig F, Cejna M, Karnel F, Gschwantler M, Ferenci P, Gangl A, Peck-Radosavljevic M. Creatinine-modifies Child-Pugh score (CPSC) compared with MELD-score to predict survival in patients undergoing TIPS. Hepatology. 2002;36:378A. [Cited in This Article: ] |
| 19. | Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 390] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13220] [Cited by in F6Publishing: 13268] [Article Influence: 368.6] [Reference Citation Analysis (0)] |
| 23. | Everson GT. MELD: the answer or just more questions? Gastroenterology. 2003;124:251-254. [PubMed] [Cited in This Article: ] |
| 24. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1825] [Cited by in F6Publishing: 1766] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 25. | Salerno F, Merli M, Cazzaniga M, Valeriano V, Rossi P, Lovaria A, Meregaglia D, Nicolini A, Lubatti L, Riggio O. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36:494-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Schepke M, Roth F, Fimmers R, Brensing KA, Sudhop T, Schild HH, Sauerbruch T. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |