Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2792
Revised: October 19, 2004
Accepted: December 8, 2004
Published online: May 14, 2005
AIM: To evaluate the clinical outcome and cost-effectiveness of transcatheter arterial ethanol-lipiodol embolotherapy on hepatocellular carcinoma (HCC).
METHODS: One hundred patients with HCC who were treated only by lobar or segmental transarterial embolization (TAE) with ethanol-lipiodol mixture were enrolled in this study. The 1st- and 2nd-year survival rates were analyzed to evaluate the feasibility of its method. These outcomes of our patients were individually correlated to the Child-Pugh classification and the computed tomographic features of HCC.
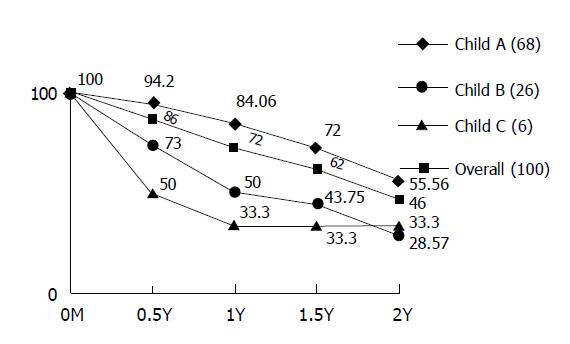
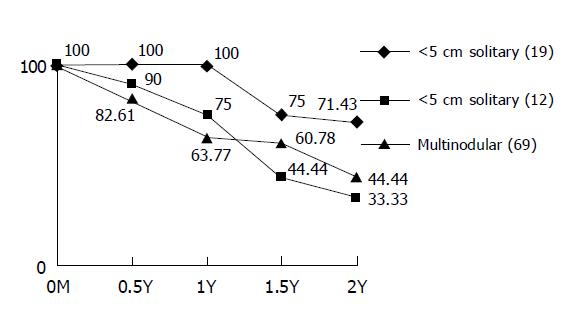
RESULTS: The overall 1st- and 2nd-year survival rates were 72% and 46%, respectively. The patients were classified into three groups according to their liver function status: 68 patients as Child-Pugh class A, 26 as Child B, and 6 as Child C. Child A had better survival rate than the Child B and/or C. The 1st-year survival rates of patients with Child A-C were 84%, 50%, and 33.3% respectively and the 2nd-year survival rates were 55.5%, 28.5%, and 33.3%, respectively. According to the computed tomographic features, solitary HCC with maximum diameter less than 5 cm had the best outcome with the 1st-year survival rate of 100% and the 2nd-year survival rate of 71.4%, while solitary HCC with maximum diameter over 5 cm and multiple HCC had the 1st-year survival rates of 75% and 63.7%, respectively, and the 2nd-year survival rates of 33.3% and 44.4%, respectively. Only one patient was complicated with abscess formation and was cured with antibiotic therapy. No mortality resulted from the procedures performed.
CONCLUSION: TAE with ethanol-lipiodol mixture is an economic, safe and feasible method for treating HCC, especially for the patients with smaller solitary HCC or with liver function status of Child-Pugh class A.
- Citation: Cheung YC, Ko SF, Ng SH, Chan SC, Cheng YF. Survival outcome of lobar or segmental transcatheter arterial embolization with ethanol-lipiodol mixture in treating hepatocellular carcinoma. World J Gastroenterol 2005; 11(18): 2792-2795
- URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2792.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2792
Hepatocellular carcinoma (HCC) had been documented as the leading cause of cancer death in our country for years[1]. Various methods of treatment including surgical and non-surgical therapies have been attempted. The non-surgical ones include transarterial embolization (TAE), transarterial chemoembolization (TACE), percutanous ethanol injection, radiofrequency ablation, systemic chemotherapy, hormone therapy, immunotherapy, and radiotherapy. Among them, surgery is traditionally believed to be the best treatment, offering the chance of complete cure by tumor removal[2]. Unfortunately, most of the HCC are unresectable at discovery, with poor survival rate of less than 6 mo[2]. On the other hand, the recurrence of HCC is high in a cirrhotic liver secondary to infectious hepatitis.
In those cases with cirrhotic liver, deteriorating liver function is a common risk factor for undertaking a large area resection. An optimal therapy with safe procedure and minimal interruption on liver function is essential. Under such circumstances, minimal invasive nonsurgical management rather than invasive surgical approach may be the choice of therapy, in which TACE is one of the most commonly used methods. However, chemotherapeutic drugs are expensive and hepatic toxic. In this study, we report the survival outcomes of TAE of HCC using the less toxic ethanol-lipiodol liquid mixture, in order to evaluate the feasibility of this embolization method. To our knowledge, this is the first report to document the therapeutic results of TAE with ethanol-lipiodol liquid mixture in the patients with inoperable HCC.
Reviewing the records of transcatheter arterial embolization (TAE) performed in our department from January 2000 to December 2002, we enrolled 100 HCC patients who received lobar or segmental TAE with the mixture of absolute ethanol and lipiodol. There were 70 males and 30 females, ranging from 39 to 82 years (mean: 63.62±9.65 years). The technique of TAE was standardized, following the set up protocol since January 2000 in our department. In order to obtain an absolute therapeutic outcome of ethanol-lipiodol TAE, the patients that underwent other treatments such as percutaneous ethanol injection, radiofrequency therapy or TACE were excluded from our study. Those HCCs with the radiographic evidence of vascular shunts or with portal vein thrombosis were also eliminated.
All patients were requested to sign consents after full explanation. With the coaxial angiographic technique, a French-3 microcatheter was selectively catheterized beyond the lobar or segmental branch of the hepatic artery supplying the HCC. Absolute ethanol (99.5%) and lipiodol were mixed in emulsion as the embolizer for TAE. The composition of absolute alcohol and lipiodol was kept at 3 to 1 ratio. However, the amount of lipiodol used was proportional to the maximum diameter of targeted HCC measured on computed tomogram (CT). If the maximum diameter was 5 cm, the amount of lipiodol used would be 5 mL. In cases of multiple HCC, the amount of lipiodol (mL) used was adjusted according to the sum of maximum diameters of all targeted HCC in centimeters. However, the maximum amount of lipiodol was always limited to 10 mL.
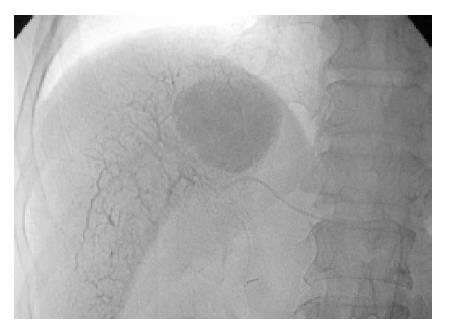
Before starting the infusion of embolizing agents, each 25 mg Demoral was given intravenously and intramuscularly, respectively. To avoid the vasospasm, intra-arterial slow injection of 3 mL of 2% lidocaine diluted with 10 mL saline were routinely administered. With the stable vital signs continuously being monitored by pulse oximeter, the ethanol-lipiodol mixture was connected to an adjustable pumping machine and was slowly infused with a set rate at 0.5-1 mL per minute. The TAE would be finished when the infusion of embolizing mixture had been completed. In another situation, the embolization would also be terminated when the retrograde filling of the portal vein was observed (Figure 1). Finally, absorbable gelfoam particles were routinely administered until sluggish inflow of the contrast medium in the supplied arteries of the embolized HCC, in order to delay the washout of the embolizers.
According to the findings on triphasic CT, the HCCs of our patients were classified into three categories. They included: (1) solitary HCC with maximum diameter less than 5 cm, (2) solitary HCC with maximum diameter greater than 5 cm, and (3) multiple HCCs of multinodular, infiltrating or mixed type. The patients were also classified into three other groups as Child A-C, according to their liver function status. We individually correlated the CT presentations of HCC and the Child-Pugh classification to the 1st- and 2nd-year survival outcomes.
Totally, 245 TAE procedures were successfully performed. Some cases of mild abdominal distension and mild fever were observed after the procedure, but all subsided within 24 h after the procedures. No major complications were immediately noticed during the procedures except one liver abscess (1%) was found 5 d after the TAE. The abscess was subsequently cured by antibiotic therapy. Based on the finding of follow-up CT, no bilioma was complicated. There was no procedure-related mortality.
According to the serum data, all our patients were hepatitis-infected, including 56 patients with viral hepatitis-B, 34 with viral hepatitis-C, six with both viral hepatitis B and C, and four with non-B and non-C viral hepatitis. At the time of TAE, 68 of our 100 patients were classified as Child-Pugh class A, 26 as Child B and six as Child C. According to the morphologic presentations of HCC, multiple HCCs were found in 69 patients, solitary HCC with maximum diameter less than 5 cm was found in 19 patients, and that greater than 5 cm was found in the remaining 12 patients.
Post-TAE serum levels of aspartate aminotransferase and alanine aminotransferase on the 3rd day increased in 195 procedures, of which an increment ranging from 50 to 300 IU/L was found in 123 procedures, ranging from 301 to 500 IU/L in 50 procedures, and >500 IU/L in the other 22 procedures. Slight elevation of the total bilirubin and a decrement in albumin levels were also noted in about 20% of our 245 TAE procedures. All these abnormal parameters returned to the initial levels within 2 wk. At that time, considerable decrement of alpha fetoprotein was noted in 172 of our TAE procedures. The alpha fetoprotein of our patients with HCCs with maximum diameter less than 5 cm was invariably improved. On the follow up triphasic CT after the first TAE, a complete package of embolized HCC without evidence of viable cancer was found in 73 patients (12 patients of HCC with maximum diameter less than 5 cm, 13 patients of HCC with maximum diameter greater than 5 cm, and 48 patients of multiple HCC). Residual viable tumors were noted in the other 27 patients (23 patients with multiple HCC and four with HCC larger than 5 cm of maximum diameter). In this series, in order to treat residual viable tumor (n = 27), local recurrence at the embolized areas (n = 8), or newly developed HCC in the otherwise liver areas (n = 46), 54 patients needed repeated TAE. Seventeen patients underwent second TAE and the other 37 patients underwent second and third TAEs.
The benefits of ethanol-lipiodol TAE compared with TACE was assessed in the cost of embolizers and the duration of hospitalization. In our country, the cost of Doxorubicin in a single TACE procedure was $US 60 for 20 mg. However, the cost of the 99.5% ethanol that we used in a single TAE procedure was $US 0.05. On the other hand, all patients were discharged from hospital about 3 d after TAE procedures. Only one patient was admitted again due to the complication of liver abscess 5 d after TAE and required hospitalization for antibiotic treatment for 8 d. No additional systemic complication prolonging the hospitalization was noticed.
The overall 1st- and 2nd-year survival rates were 72% and 46%, respectively. The 1st-year survival rate distinctively varied to Child-Pugh classification with 84% for Child A, 50% for Child B and 33.3% for Child C. For the 2-year survival rate, Child A was still the best but the rate decreased to 55.56%. The 2-year survival rate of Child B and C were close to the survival rate of 28.57% and 33.3%, respectively (Figure 2). The cause of the same 1st- and 2nd-year survival rate (33.3%) of our Child C patients was that two of our six Child C patients had solitary HCC less than 5 cm and were well controlled by TAE in the period of 2 years.
The survival rates of patients with solitary HCC less than 5 cm, and that greater than 5 cm and multiple HCCs were 100%, 75%, and 63.77%, respectively for the 1st year and 71.43%, 33.33%, and 44.44%, respectively for the second year (Figure 3). The patients died of the uncontrollable progression of the disease including the decompensation of liver function in 18 patients, widespread dissemination of HCCs in 25, and the development of portal vein thrombosis in 9.
In Taiwan, most patients with HCC were cirrhotic related, accompanied by liver dysfunction. The liver function impairment or the HCC involvement often limit the surgery even though the survival rate of surgical cases is believed to be significantly higher than non-surgical cases[3]. However, the survival rate had been improved by the progressive advances of angiographic techniques. With the development of microcatheter system, we are able to superselectively catheterize a small angiocatheter into the tumor-feeding artery in order to achieve the lobar or segmental targeting embolization while avoiding arterial injury. With such a high technical performance, the embolizers can be effectively injected into HCC tumor beds.
Since the application of TAE on unresectable HCC, several reports have documented the effectiveness of this treatment on unresectable HCC[4-7]. Various embolizers had been introduced. TACE was currently used in most centers due to the combined therapeutic effects of tumor chemotoxicity and ischemia. In a report of a Chinese population sample of inoperable HCC with Child A and B, the 1st- and 2nd-year survival rate after TACE were 86.3% and 78.8% respectively[8]. In our result, the survival rates of the patients with Child A and B were 84% and 50%, respectively for the 1st year, and 55.5% and 28.5% respectively for the 2nd year. The survival rate of our patients with Child C maintained at 33.3% for the 1st- and 2nd-year survival rates, however, due to small populations in this group, such survival rate necessitated further investigation with a larger series.
In 1983, sonographically guided percutanous ethanol injection therapy (PEIT) was introduced as a new alternative approach in treating small HCC[9]. This method was subsequently agreed upon by many clinicians on the point of effectiveness in treating a small HCC[10-13]. For a large HCC, PEIT has difficulties to fill the entire tumor tissue because of the complicated architecture of septa and inhomogeneous infiltration of ethanol within the tumor. The combined treatment with PEIT and TACE has been reported as effective in causing complete tumor necrosis[12-14]. The capsular invasion or small daughter nodules can also be treated[12-14]. The reported 1st- and 2nd-cumulative survival rates of combined therapy with PEIT and TACE were 78% and 54%, respectively[15]. Such a result was similar to ours with ethanol-lipiodol TAE.
Currently, TAE or TACE are the mainstream in the treatment of unresectable HCC. Since chemotherapeutic drugs are hepatotoxic, we use a less toxic ethanol as an embolizer in order to preserve the liver function as much as possible. Ethanol is a traditionally cheap and safe liquid embolizer that has been used to treat vascular lesions or tumors. In 1997, the embolization effect of ethanol on HCC was established on the basis of animal models[16,17]. Kan et al[18], reported the pathways of iodized oil and silicon rubber solution shunting from the hepatic artery to the portal vein, and the mechanism of sinusoid embolization by iodized oil[19]. On the other hand, two reports have documented the clinical efficacy of this ethanol-lipiodol TAE on HCC[20,21]. The results showed successful liver lobar ablation with only minimal side effects.
From the fluoroscopic observation on an animal model, dual embolization will be induced by slow infusion of insoluble substance, such as a mixture of ethiodol and ethanol, which appears as small droplets passing through the hepatic sinusoids (the bridges between the hepatic artery and the portal vein) to the portal vein. This achieves complete thrombotic effect with embolizers package in both the arteries supplying the tumor and its adjacent parenchymal portal veins[16,21]. A slow infusion of embolizers is the key point for the success of dual embolization. The slow infusion technique not only prevents the spasmodic change of the feeding artery, but also allows the embolizers to flow primarily to the fast stream tumor vessels. Once the tumor vessels have been filled up, the liquid, insoluble embolizers will then start to deposit along the feeding hepatic artery and the parenchymal portal veins.
In our results, tumor morphologic features and patient liver function status affected the survival rates of HCC patients after TAE with ethanol-lipiodol mixture. Better survival rates were observed in patients with solitary smaller HCC or with liver function status of Child A. The outcome of our study was similar to that of traditional TACE. Nevertheless, ethanol-lipiodol TAE is a cheap and less toxic alterative treatment feasible for HCC.
We sincerely thank Miss Stephanie Cheung, a student of Cell Biology and Genetics at the University of British Columbia, Canada for manuscript preparation.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Lin TM, Chen CJ, Tsai SF, Tsai TH. Hepatoma in Taiwan. J Natl Public Health Assoc. 1998;8:91. |
| 2. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 3. | Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4:3S-6S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453-456. [PubMed] |
| 6. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 673] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Wheeler PG, Melia W, Dubbins P, Jones B, Nunnerley H, Johnson P, Williams R. Non-operative arterial embolisation in primary liver tumours. Br Med J. 1979;2:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Yuen MF, Chan AO, Wong BC, Hui CK, Ooi GC, Tso WK, Yuan HJ, Wong DK, Lai CL. Transarterial chemoembolization for inoperable, early stage hepatocellular carcinoma in patients with Child-Pugh grade A and B: results of a comparative study in 96 Chinese patients. Am J Gastroenterol. 2003;98:1181-1185. [PubMed] |
| 9. | Sugiura N, Takara K, Ohto N. Percutaneous intratumoral injection of ethanol under ultrasound imaging for treatment of small hepatocellular carcinoma. Acta Hepatol Jpn. 1983;24:920. |
| 10. | Fujimoto T. The experimental and clinical studies of percutaneous ethanol injection therapy (PEIT) under ultrasonography for small hepatocellular carcinoma. Acta Hepatol Jpn. 1988;29:52-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Ebara M, Ohto M, Sugiura N, Kita K, Yoshikawa M, Okuda K, Kondo F, Kondo Y. Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol. 1990;5:616-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, Niwa Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Tanaka K, Okazaki H, Nakamura S, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Yamamoto K, Masuzawa M, Kato M, Kurosawa K, Kaneko A, Ishida H, Imamura E, Park NJ, Shirai Y, Fujimoto K. Evaluation of combined therapy with chemoembolization and ethanol injection for advanced hepatocellular carcinoma. Semin Oncol. 1997;24:S6-50-S6-S6-50-55. [PubMed] |
| 15. | Li YH, Wang CS, Liao LY, Wang CK, Shih LS, Chen RC, Chen PH. Long-term survival of Taiwanese patients with hepatocellular carcinoma after combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection. J Formos Med Assoc. 2003;102:141-146. [PubMed] |
| 16. | Kan Z, Wallace S. Transcatheter liver lobar ablation: an experimental trial in an animal model. Eur Radiol. 1997;7:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ito K, Kusunoki H, Okamoto E, Ozawa M, Ishikawa A, Matsuura M, Nakajima N. Intra-arterial alcoholization of advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Kan Z, Ivancev K, Lunderquist A. Peribiliary plexa--important pathways for shunting of iodized oil and silicon rubber solution from the hepatic artery to the portal vein. An experimental study in rats. Invest Radiol. 1994;29:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kan Z, Wallace S. Sinusoidal embolization: impact of iodized oil on hepatic microcirculation. J Vasc Interv Radiol. 1994;5:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Cheng Y, Kan Z, Chen C, Huang T, Chen T, Yang B, Ko S, Lee T. Efficacy and safety of preoperative lobar or segmental ablation via transarterial administration of ethiodol and ethanol mixture for treatment of hepatocellular carcinoma: clinical study. World J Surg. 2000;24:844-850; discussion 850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Park JH, Han JK, Chung JW, Choi BI, Han MC, Kim YI. Superselective transcatheter arterial embolization with ethanol and iodized oil for hepatocellular carcinoma. J Vasc Interv Radiol. 1993;4:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |