Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2739
Revised: October 19, 2004
Accepted: December 3, 2004
Published online: May 14, 2005
AIM: To illustrate the pathophysiological role of metallothionein (MT) in gastric ulcer induced by stress.
METHODS: Wistar rats underwent water-immersion-restraint (WIR) stress, ZnSO4 (an MT inducer) treatment, WIR+ZnSO4 or WIR+MT, and the ulcer index (UI) was estimated in excised stomach and liver tissues. The mRNA level of gastric MT was determined by semi-quantitative RT-PCR. The MT content in gastric and hepatic tissues was determined by Cd/hemoglobin affinity assay. The lipid peroxidation products malondialdehyde (MDA) and conjugated dienes (CD) were estimated by use of thiobarbituric acid reactive species and ultraviolet spectrophotometry.
RESULTS: WIR stress induced severe gastric mucosal lesions in rats. Compared with control rats, stressed rats had increased lipid peroxide content in serum and stomach and liver tissues. MDA content was increased by 34%, 21% and 29% and CD level by 270%, 83% and 28%, respectively. MT content in the stomach and liver was increased by 0.74- and 1.8-fold, and the MT-mRNA level in the stomach was increased by 26%. Pretreatment with ZnSO4 prevented gastric lesion development (the UI was 87% lower than that without pretreatment), and the MDA and CD content in serum and tissues was lower. The MT content in the liver was double in rats that were not pretreated, and the MT mRNA level in the stomach was 35% higher. MT administration 1 h before the WIR stress prevented gastric lesion development (the UI decreased by 47% compared with that in rats not pretreated), and the MDA and CD content in serum and tissues was significantly lower.
CONCLUSION: In WIR-stressed rats, the MT level was increased in serum and in stomach and liver tissues. Pre-administration of exogenous MT or pre-induction of endogenous MT can protect the gastric mucosa against stress-induced ulcers and inhibits the formation of stress-induced lipid peroxide. MT could have a gastroprotective effect and might be a new interventive and therapeutic target in stress-induced gastric ulcers.
- Citation: Jiang P, Chang L, Pan CS, Qi YF, Tang CS. Protective role of metallothionein in stress-induced gastric ulcer in rats. World J Gastroenterol 2005; 11(18): 2739-2743
- URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2739
Physical or psychological stress is one of the common causes of upper gastrointestinal ulceration[1]. Since Hans Selye’s report established that gastric ulcer occurs under chronic stressful conditions, a plethora of investigations have been performed to unravel mechanisms underlying stress-induced gut lesions[2]. Molecules and their receptors involved in the stress response are being characterized. Clinical observations indicate that various kinds of stress such as shock, burns, sepsis, and severe trauma as well as severe emotional distress are closely related to acute upper gastrointestinal erosions and ulcerations[3]. Although the pathogenesis of gastric lesions is not completely understood, mucosal ischemia and enhanced back diffusion of hydrogen ions have been proposed as major pathophysiological phenomenon in these stress-induced injuries. The production of oxygen-free radicals via the xanthine-xanthine oxidase system and neutrophils and lipid peroxidation initiated by the produced reactive oxygen species (ROS) have been investigated to explain the mechanisms of acute gastric lesion formation associated with stress.
Lipid peroxidation induced by oxygen-free radicals may play a critical role in the pathogenesis of acute gastric mucosal injury in experimental animals and humans[4,5]. As well, stress could agitate the injured and defensive capacity of the body. On one hand, stress can increase the injured substances; on the other, it can enhance the endogenous defense systems. So, it is important to reinforce the function of the defense system for the prevention and therapy of stress-induced diseases.
Metallothionein (MT) is a low-molecular-weight, heavy-metal-binding, cysteine-rich protein. The highest concentration of MT is found in the liver, kidney, intestine and pancreas. Called as an acute-phase protein or stress protein, MT responds to various stresses such as physiological and psychological stresses[6], and MT induced by stressors has been shown to detoxify harmful heavy metals and ROS produced by the stressors[7]. The ability of MT to capture hydroxyl radicals, which are primarily responsible for the toxicity of ROS, is 300 times greater than that of glutathione, the most abundant antioxidant in the cytosol[8]. In addition, MT has been implicated in protecting cells from stress by regulating metabolism and the immunity reaction[9].
We hypothesized that MT may play a central role as a protective substance in the development of gastric mucosal lesions in rats under stress. Therefore, we investigated the expression of MT in rats treated with a water immersion restraint (WIR) and examined the effects of exogenous MT and pre-induction of endogenous MT on the formation of stress-induced gastric ulcers. We attempted to confirm whether MT has a protective effect on the pathogenesis of gastric ulcer due to stress and to explore its mechanism.
All animal care and experimental protocols were in compliance with the Animal Management Rule of the People’s Republic of China and the Care and Use of the Laboratory Animals guide of the First Hospital, Peking University. Wistar rats weighing 180-220 g were maintained under standard conditions of temperature, humidity and light (12 h dark, 12 h light). They were provided with Purina chow and free access to water. MT (from rabbit liver), ZnSO4 (an MT inducer) and bovine serum albumin were from Sigma Chemical Co. (St. Louis, MO); 109Cd was purchased from Dupont NEN Inc. (Boston, MA); TRIzol reagent was from Gibco (Gaithersburg, MD); dNTP was from Clontech (Palo Alto, CA); and M-MuLV reverse transcriptase, Taq DNA polymerase, RNasin and oligo (dT) primer were from Promega (Madison, WI). Oligonucleotides used for amplification were synthesized by Sai Bai Sheng (Beijing, China). The MT-I sequences of oligonucleotide were MT-I sense (MT-S), 5’-GCGATCTCTCGTTGATCTCC 3’; and MT-I antisense (MT-A), 5’-CAGCTGCACTTGTCCGAAG 3’. The β-actin sequences of oligonucleotide were sense, 5’-ATCTGGCACCACACCTTC-3’; and antisense, 5’-AGCCAGGTCCAGACGCA-3’. Other chemicals were of analytical grade.
Rats were randomly divided into control, WIR, ZnSO4, WIR+ZnSO4 and WIR+MT groups (n = 6 for every group) and were starved for 24 h but were allowed free access to water. The ZnSO4 and WIR+ZnSO4 groups were injected subcutaneously with 0.8 nmol/kg ZnSO4 24 h before killing. Except for control rats, all rats were restrained in a wire cage and immersed up to the depth of the xiphoid process in a 23 °C water bath to create WIR stress[10]. MT was injected intraperitoneally in the WIR+MT rats (2.5×10-7 mol/kg) 1 h before the onset of WIR stress. Rats were killed under pentobarbital anesthesia after 6-h WIR stress. After infusion of 10 mL of saline and fixation with 40 g/L formaldehyde for 5 min, rats were cut open along the gastric greater curvature, and stomachs and livers were removed. The gastric mucosa was carefully examined for lesions linear breaks (erosions) at the mucosal surface of the glandular part under a stereoscopic microscope. The ulcer index (UI), extent of the lesion, is expressed as the sum of the length of these breaks per stomach[11].
The MT concentration in tissues was determined by use of the 109Cd/hemoglobin affinity assay[12]. Tissues from the stomach and liver were homogenized in four volumes of 10 mmol/L Tris-HCl buffer (pH 7.4), and the homogenate was centrifuged at 10000 g for 10 min at 4 °C. The supernatant was heated for 2 min in a boiling water bath, then centrifuged at 10000 g for 2 min to remove heat-precipitated proteins. A 200-μL aliquot of each column fraction or heat-denatured tissue supernatant fraction was placed in a 1.5-mL microcentrifuge tube. The samples were 10-fold to keep the MT concentration in the range of the assay. An amount of 200 μL of radioactive cadmium (2.0 mg 109CdCl2/mL with radioactivity of 1.0 μCi/mL) was added to each centrifuge tube, mixed, and incubated for 10 min at 25 °C. The free cadmium was removed by the following procedure: 100 μL of 2% bovine hemoglobin solution was added to each tube, and the tubes were heated in boiling water for 2 min, cooled in ice, and centrifuged at 10000 g for 5 min at 4 °C. This procedure was repeated twice to remove the excess radioactive cadmium. A clear supernatant fluid was transferred to a gamma counting tube. The amount of radioactivity in the last supernatant fraction was then measured by use of a gamma counter (Perkin Elmer 1470). The quantity of cadmium-binding proteins (MT equivalents) was calculated[12] and expressed as micromoles of cadmium per gram of heat stable protein.
Lipid peroxidation was estimated in terms of thiobarbituric acid reactive species with use of 1,1,3,3-treaethyloxypropane as a standard. Briefly, an aliquot of 250 µL of serum or tissue homogenates was mixed thoroughly with 2.25 mL of fresh TBA (0.67%) and heated at 95 °C for 30 min in a water bath. The suspension was then cooled to room temperature, centrifuged at 3000 r/min for 10 min, and the pink colored supernatant was taken for spectrophotometry measurement at 532 nm for MDA assay. MDA content was expressed in terms of nanomoles per liter for serum and nanomoles per gram wet weight of tissue[13].
Lipid peroxidation measurement by CD level was performed as described[14]. An aliquot of 500 µL of serum and tissue homogenates was extracted in chloroform-methanol (2:1) and dried with N2. The dried extract was re-dissolved in cyclohexane and absorbency measured at 234 nm by use of a spectrophotometer.
Total RNA of gastric tissue was prepared by in situ lysis of the tissues with TRIzol reagent. One microgram total tissue RNA was reverse-transcribed into single-strand cDNA with use of M-MuLV reverse transcriptase and oligo (DT) 15 primers. PCR was performed in a 0.2-mL tube containing tissue cDNA (2 μL), 5 μmol/L per each MT-S and MT-A primer mixture (1 μL), 2.5 mmol/L per each dNTP mixture (1 μL), 1.5 mmol/L MgCl2 (1.5 μL), 10×PCR buffer (2.5 μL), and 1.25 unit Taq DNA polymerase, in a total volume of 25 μL. After denaturing at 95 °C for 5 min, PCR was run at 94 °C for 30 s, 61 °C for 30 s, and 72 °C for 40 s for 30 cycles (72 °C for 5 min). A total of 8 μL of PCR product was separated in a 1.5% agarose gel, and stained with ethidium bromide. The optical density of the 256-bp band was measured with use of the Gel Documentation System (Bio-Rad, Hercules, CA). The amplification of MT cDNA was confirmed by digestion of the PCR products with restriction enzyme BglII. An amount of 2 μL PCR product was amplified again with the two rat β-actin primers at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 40 s for 24 cycles (72 °C for 5 min), and the optical density of the β-actin band (291 bp) was measured. The ratio of MT mRNA to β-actin mRNA was considered as the relative amount of MT mRNA.
All data are expressed as mean±SD. Statistical differences were evaluated by one-way ANOVA and then by the Student-Newman-Keuls test; comparisons between two groups involved the use of Student’s t-test. Linear regression analysis was used to assess the correlation between variables. A value of P<0.05 was considered statistically significant.
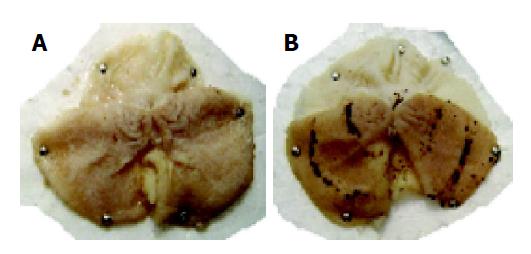
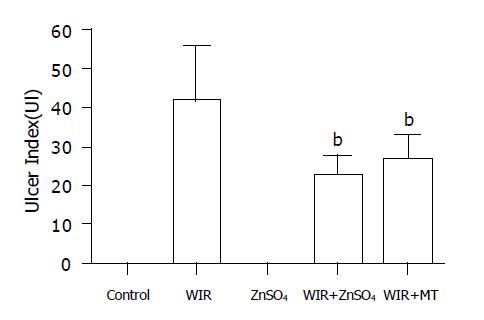
Gastric mucosal lesions developed in rats subjected to WIR stress over 6 h, as shown in Figure 1. Macroscopic observation showed lesions, most often 1-2 mm in size, or petechial bleeding. The area of involvement was confined to the glandular part of the stomach. The mean UI was 42.8±11.8 (Figure 2). In contrast, control rats had no lesions. As shown in Table 1, lipid peroxide content in the gastric mucosa of stressed rats was higher than that of the control rats. Compared with control rats, stressed rats showed increased MDA content in the serum and stomach and liver tissues, by 34%, 21% and 29%, respectively (all P<0.01), and increased CD content, by 270%, 83% and 28%, respectively (all P<0.01, Table 1).
| Control | WIR | ZnSO4 | WIR+ ZnSO4 | WIR+MT | |
| CD content in plasma (A/mL) | 10.19±1.81 | 37.37±2.04b | 10.33±2.07a | 19.08±1.52ab | 18.64±1.32ab |
| CD content in stomach (A/g tissue) | 39.96±6.74 | 73.09±3.71b | 51.39±4.41ab | 60.48±3.93ab | 62.43±4.99ab |
| CD content in liver (A/g tissue) | 64.87±3.05 | 83.26±3.78b | 66.99±4.7a | 68.07±4.93a | 80.76±3.16b |
| MDA content in plasma (nmol/mL) | 3.65±0.41 | 4.88±0.30b | 3.98±0.35a | 4.20±0.21a | 4.77±0.18b |
| MDA content in stomach (nmol/g tissue) | 4.17±0.18 | 5.06±0.77 | 4.67±0.54 | 4.21±0.62 | 4.01±0.32a |
| MDA content in liver (nmol/g tissue) | 6.86±0.86 | 8.87±1.20b | 7.27±0.57a | 6.60±0.87a | 7.21±0.44a |
| MT content in stomach (mg/g protein) | 1.080±0.066 | 1.875±0.285c | 1.925±0.7ce | 1.813±0.448ce | 1.493±0.188c |
| MT content in liver (mg/g protein) | 9.955±1.358 | 28.325±7.117 | 54.165±19.224ce | 56.63±15.218ce | 12.575±6.106c |
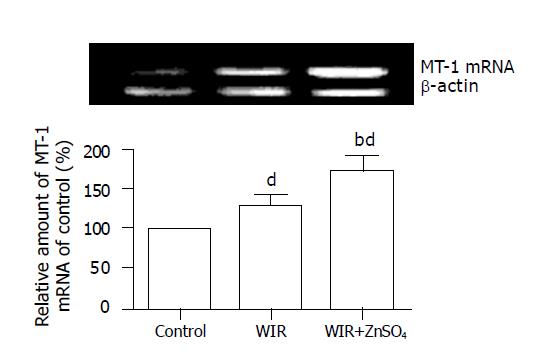
Compared with control rats, stressed rats showed increased MT content, by 0.74- and 1.8-fold (all P<0.01), in the stomach and liver, respectively; the MT-I mRNA level in the stomach was increased by 26% (P<0.01, Figure 3).
MT content in the stomach and liver of ZnSO4-treated rats was increased by 74% and 180% compared with that in control rats (all P<0.01). MT was generated effectively in the WIR+ZnSO4 group and attenuated gastric lesion development (with an 87% lower UI than that of WIR-alone rats; P<0.01). Compared with WIR-alone rats, the WIR+ZnSO4-treated rats showed double the MT content in hepatic tissues (P<0.01), and the MT-I mRNA level in the stomach was increased by 35% (P<0.01). Pretreatment with ZnSO4 also ameliorated the increased level of lipid peroxidation products induced by WIR stress in serum, and stomach and liver tissues, decreased the MDA content, by 14% (P<0.05), 16% (P<0.05) and 26% (P<0.01), respectively, and decreased the CD content, by 49% (P<0.01), 18% (P<0.05) and 18% (P<0.05, Table 1).
MT pretreatment not only prevented gastric lesion development (the UI decreased by 47% compared with that in WIR-alone rats; P<0.01) but also inhibited the formation of MDA and CD. Compared with WIR-alone rats, MT-pretreated rats showed decreased MDA content in the stomach and liver, by 20% (P<0.05) and 19% (P<0.05), respectively, and decreased CD content in serum and stomach, by 15% (P<0.05) and 49% (P<0.01, Table 1), respectively.
It is well known that oxygen-free radicals, primarily superoxide anion (O2-.) and hydroxyl radical (OH.), play an important role in the pathogenesis of acute gastric lesions induced by experimental stress[15]. Such radicals cause tissue injury through lipid peroxidation. ROS can induce cellular membrane damage by peroxidation of phospholipid fatty acids. In rats subjected to WIR stress, the development of gastric mucosal lesions is closely related to the enhanced formation of lipid peroxide and enhanced oxidation of nonprotein sulfhydryl and nonprotein SH, which depends on an increased generation of oxygen-free radicals such as superoxide anion via the xanthine-xanthine oxidase system[16-18].
MT was first isolated from horse kidney and characterized more than 40 years ago[19]. Typically, MT has low molecular weight (<7000 Da). There are four known isoforms. The MT-I and MT-II isoforms have a ubiquitous tissue distribution and are abundant in the liver, pancreas, gastrointestinal system and kidney, whereas MT-III and MT-IV are restricted to the brain and skin. The MT-I and MT-II isoforms are inducible by various stress conditions and compounds, including ROS, glucocorticoids, cytokines, and metal ions. In contrast, the MT-III and MT-IV isoforms are relatively unresponsive to these inducers.
Several lines of evidence suggest that MT can protect cells from damage caused by agents. For example, overexpression of MT in cells diminishes the sensitivity of the cells to compounds that generate oxygen-free radicals, DNA-damaging agents such as UV radiation, and nitric oxide, whereas targeted disruption of the MT gene renders the cells significantly more sensitive to stress damage[20,21]. MT-I overexpression provides protection against ischemia-reperfusion injury of the heart, and pre-induction of hepatic MT by Zn can diminish hepatic injury by lipid peroxidation[22]. These results suggest that MT could play a central role in protecting cells against oxidative stress. Growing experimental evidence suggests that lipid peroxidation is an important pathogenetic factor of gastric mucosal lesions induced by stress[15]. However, the alteration of MT generation and its role during the pathogenesis of stress-induced gastric ulcer is unclear.
The present study has shown that rats treated with WIR stress showed greater development of gastric ulcer. Gastric mucosal injury, or stress ulceration, arose in conscious animals in response to psychological stress from immersion in water with physical restraint. The most common group of indices used to assess oxidative stress is peroxidation products of lipids, usually polyunsaturated fatty acids, which are susceptible to attack by free radicals. The initial products of lipid peroxidation are CD hydroperoxides. These active substances decompose into various aldehydes or, if the original fatty acid is arachidonic acid, isoprostanes. All of these products of degradation and decomposition (CD and the widely used end product MDA) are used in assessing oxidative stress. In the present study, rats subjected to WIR stress for 6 h showed significantly increased levels of MDA and CD in serum and gastric and hepatic tissues.
As shown in our experiment, in WIR-stressed rats, the MT level in the stomach and liver was significantly increased and the MT mRNA expression up-regulated. ZnSO4, a strong inducer of MT, markedly enhanced the MT generation in gastric and hepatic tissues and up-regulated MT mRNA expression. Moreover, pre-treatment with ZnSO4 ameliorated the generation of stress-induced gastric ulcer and reduced the levels of lipid peroxide (MDA and CD) in the serum and gastric and hepatic tissues. Exogenous MT administered 1 h before WIR stress effectively suppressed the generation of gastric ulcer[23] and significantly decreased the formation of lipid peroxidation products. These results indicate that MIR stress-induced endogenous MT generation, and endogenous MT pre-induced or supplemented inhibited the formation of lipid peroxide and gastric ulcer. MT could be a protective factor against stress-induced gastric mucosal injury. Other researchers[24,25] treated the mice with ethanol or pylori, and they found that the gastric lesion indices were significantly higher in MT-null mice than in wild-type mice; these results suppose that MT may play a cytoprotective role under these conditions.
MT is an acute-phase protein induced by various stressors. The MT gene promoter has response elements to heavy metals (MRE), glucocorticoids (GRE), oxidative agents, electrophilic compounds and xenobiotics (ARE). The expression and synthesis of MT are induced for heavy metals (Zn, Cu, Cd and also Hg, Pb, As, Ni, Ag), glucocorticoids and other stress hormones, cytokines, and free radicals, as well as UV and ionizing radiation. Zn and Cu are physiological inductors of MT. Other inductors act more or less actively as stress agents. Receiving the earliest attention as regulators of MT expression, glucocorticoids are responsible in part for augmenting hepatocyte zinc pools during such stimulation. Two glucocorticoid response-element sequences are 17 kb upstream in the 5’ flanking region of the mouse MT promoter. WIR stress promptly enhances the release of glucocorticoid hormone[26]. MT is known to be the strongest endogenous scavenger for hydroxyl radicals, and kinetic study in vitro demonstrated that MT was 38.5-fold more potent than glutathione in preventing hydroxyl radical-generated DNA degradation[27].
Stress is a two-edged sword for the body: it can agitate the injury and defensive capacity of the body. So it is necessary to reinforce the function of the endogenous defensive system to prevent and treat disease. As a member of the endogenous defensive system, MT might play an important role in the pathogenesis of stress. It is worth investigating further whether MT could be a new target in preventing and treating gastric ulcer.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Spirt MJ. Stress-related mucosal disease: risk factors and prophylactic therapy. Clin Ther. 2004;26:197-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 2. | Tryba M, Cook D. Current guidelines on stress ulcer prophylaxis. Drugs. 1997;54:581-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Guldvog I. Stress ulceration: possible pathogenic mechanisms. Scand J Gastroenterol Suppl. 1984;105:9-13. [PubMed] |
| 4. | Bhattacharjee M, Bhattacharjee S, Gupta A, Banerjee RK. Critical role of an endogenous gastric peroxidase in controlling oxidative damage in H. pylori-mediated and nonmediated gastric ulcer. Free Radic Biol Med. 2002;32:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Kwiecień S, Brzozowski T, Konturek PC, Pawlik MW, Pawlik WW, Kwiecień N, Konturek SJ. Gastroprotection by pentoxyfilline against stress-induced gastric damage. Role of lipid peroxidation, antioxidizing enzymes and proinflammatory cytokines. J Physiol Pharmacol. 2004;55:337-355. [PubMed] |
| 6. | Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 916] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 7. | Kondoh M, Kamada K, Kuronaga M, Higashimoto M, Takiguchi M, Watanabe Y, Sato M. Antioxidant property of metallothionein in fasted mice. Toxicol Lett. 2003;143:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004;279:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Mocchegiani E, Giacconi R, Muti E, Rogo C, Bracci M, Muzzioli M, Cipriano C, Malavolta M. Zinc, immune plasticity, aging, and successful aging: role of metallothionein. Ann N Y Acad Sci. 2004;1019:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Takagi K, Okabe S. The effects of drugs on the production and recovery processes of the stress ulcer. Jpn J Pharmacol. 1968;18:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 246] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Holzer P, Guth PH. Neuropeptide control of rat gastric mucosal blood flow. Increase by calcitonin gene-related peptide and vasoactive intestinal polypeptide, but not substance P and neurokinin A. Circ Res. 1991;68:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Eaton DL, Toal BF. Evaluation of the Cd/hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol Appl Pharmacol. 1982;66:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 362] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18812] [Article Influence: 409.0] [Reference Citation Analysis (0)] |
| 14. | Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 1995;47:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Yasukawa K, Kasazaki K, Hyodo F, Utsumi H. Non-invasive analysis of reactive oxygen species generated in rats with water immersion restraint-induced gastric lesions using in vivo electron spin resonance spectroscopy. Free Radic Res. 2004;38:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Sung JJ. The role of acid suppression in the management and prevention of gastrointestinal hemorrhage associated with gastroduodenal ulcers. Gastroenterol Clin North Am. 2003;32:S11-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Uehara K, Miura S, Takeuchi T, Taki T, Nakashita M, Adachi M, Inamura T, Ogawa T, Akiba Y, Suzuki H. Significant role of ceramide pathway in experimental gastric ulcer formation in rats. J Pharmacol Exp Ther. 2003;305:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kwiecień S, Brzozowski T, Konturek PC, Pawlik MW, Pawlik WW, Kwiecień N, Konturek SJ. The role of reactive oxygen species and capsaicin-sensitive sensory nerves in the pathomechanisms of gastric ulcers induced by stress. J Physiol Pharmacol. 2003;54:423-437. [PubMed] |
| 19. | Kojima Y, Berger C, Vallee BL, Kägi JH. Amino-acid sequence of equine renal metallothionein-1B. Proc Natl Acad Sci USA. 1976;73:3413-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 143] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Haq F, Mahoney M, Koropatnick J. Signaling events for metallothionein induction. Mutat Res. 2003;533:211-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 537] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 22. | Nath R, Kumar D, Li T, Singal PK. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology. 2000;155:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Mimura T, Tsujikawa K, Yasuda N, Nakajima H, Haruyama M, Ohmura T, Okabe M. Suppression of gastric ulcer induced by stress and HCL-ethanol by intravenously administered metallothionein-II. Biochem Biophys Res Commun. 1988;151:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Takano H, Satoh M, Shimada A, Sagai M, Yoshikawa T, Tohyama C. Cytoprotection by metallothionein against gastroduodenal mucosal injury caused by ethanol in mice. Lab Invest. 2000;80:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Tran CD, Huynh H, van den Berg M, van der Pas M, Campbell MA, Philcox JC, Coyle P, Rofe AM, Butler RN. Helicobacter-induced gastritis in mice not expressing metallothionein-I and II. Helicobacter. 2003;8:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Filaretova LP, Podvigina TT, Bagaeva TR, Tanaka A, Takeuchi K. Mechanisms underlying the gastroprotective action of glucocorticoids released in response to ulcerogenic stress factors. Ann N Y Acad Sci. 2004;1018:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Potts RJ, Bespalov IA, Wallace SS, Melamede RJ, Hart BA. Inhibition of oxidative DNA repair in cadmium-adapted alveolar epithelial cells and the potential involvement of metallothionein. Toxicology. 2001;161:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |