Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2653
Revised: May 28, 2004
Accepted: June 17, 2004
Published online: May 7, 2005
AIM: To evaluate the expression of apoptosis related gene Fas ligand (FasL) in human hepatocellular carcinoma (HCC) cells HepG2 and its significance in apoptosis.
METHODS: Levels of soluble Fas ligand (sFasL) in a group of patients with hepatitis B virus (HBV)-induced chronic hepatitis, HBV-positive liver cirrhosis and HCC were evaluated. In a further study, the recombinant eukaryotic expression plasmid pcDNA3.1hisB-FasL was transfected into HCC cells HepG2 by lipofection, and then soluble FasL was examined in the supernatant of culture cells by EIA, FasL expression in HepG2 cells was detected by immuohistochemistry. After being stained by annexin V and propidium iodine, cells were passed through a flow cytometer and examined by a fluorescence microscope and a laser scanning microscope.
RESULTS: The sFasL levels were significantly lower in patients with HCC when compared to the patients with hepatitis or liver cirrhosis. In comparison with untransfected cells, the soluble FasL could be detected in the supernatant of transfected cells. FasL was expressed on the membranes and cytoplasm of transfected cells. The apoptotic cell rate was 36.30% in transfected cells, and was 11.53% in untransfected cells. Moreover, the different stage of apoptotic cells could be distinguished by annexin V and propidium iodine staining.
CONCLUSION: Fas ligand is an apoptotic pathway of HCC cells.
- Citation: Chen J, Su XS, Jiang YF, Gong GZ, Zheng YH, Li GY. Transfection of apoptosis related gene Fas ligand in human hepatocellular carcinoma cells and its significance in apoptosis. World J Gastroenterol 2005; 11(17): 2653-2655
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2653
Apoptosis is a cellular suicide program that is a regular, non-necrotic form of cell death. Apoptosis in epidermal cell can be regulated by a variety of signals of which the Fas/Fas ligand (Fas/FasL) pathway is one of the best-described signals[1-3]. Fas is a transmembrane receptor in the TNF receptor family[4,5] that transduces an apoptotic signal into cells when cross-linked by FasL. The ligand for Fas is expressed by intraepithelial lymphocytes and lamina propria lymphocytes. Lymphocytes expressing FasL are not dependent on either classical or nonclassical major histocompatibility complex restriction but recognize cells expressing Fas[6]. It has been shown that hepatoma cells could express Fas and FasL. Several lines of evidence suggest that Fas/FasL interactions are important in immune-epithelial communication in the liver[7,8]. Recent researches have shown epithelial cells can be apoptotic by self-secretion or side secretion of Fas and FasL without involvement of immune cells[9-13].
Liver cancer is common in China, and its treatment is difficult. To investigate the mechanism of carcinogenesis of liver cancer would facilitate its treatment.
Soluble Fas ligand was tested by EIA (MBL, USA) in a group of patients with hepatitis B virus (HBV)-induced chronic hepatitis (12 patients), HBV-positive liver cirrhosis (6 patients) and hepatocellular carcinoma (HCC,16 patients), six volunteers served as normal controls.
Human FasL was amplified by PCR from pBluescript SK (gift from S Nagata, Osaka Bioscience Institute, Japan) using the following oligonucleotides 5’-CCTCTACAGGAC-TGAGAAGAAG-3’ and 5’-CAACATTCTCGGTGCCTG-TAAC-3’. PCR products were excised using EcoRI and BamHI and ligated into the mammalian expression vector pCDNA3.1hisB (Invitrogen). The resulting pCDNA31hisB-hFasL plasmid was purified using the Qtip500 system (Qiagen). The correct insertion of hFasL was verified by PCR and a sequence analysis system (BioSino, Shanghai, China).
HepG2 cells (stored in Xiangya cell center, Changsha, China) and HepG2.2.15 cells (gift from South Hospital, the First Military Medical University, Guangzhou, China) were cultured at 37 °C containing 50 mL/L CO2 in Dulbecco’s modified Eagle’s medium and 10 mL/L fetal calf serum supplemented with pencillin-streptomycin and fungizone (GIBCO, Inc.). All transfections were performed using the lipofection system (GIBCO, Inc.). Then the transfected HepG2 cells were cultured with HepG2.2.15 cells.
Twenty-four and 48 h after, the supernatant and cultured HepG2 cells were harvested, soluble FasL was detected using an EIA human FasL detect kit (MBL, USA). HepG2 cells were stained by S-P using a S-P human FasL detect kit (Maixin Bio, Fuzhou, China).
Forty-eight hours after the HepG2 and HepG2.2.15 cells were cocultured and stained by annexin V and propidium iodine (purchased from Boehringer Mannheim, Germany) and then examined under a fluorescence microscope or a laser scanning microscope to identify the morphological changes of apoptosis. Stained cells were washed twice with PBS, dispersed, filtered through a 360 mesh nylon net to make single cell suspension. 3×109 cells/L were detected by a flow cytometer (FACSort, B-D Co., USA) fluorescent intensity was detected using exciting light 488 nm and emission light 515 nm. The scatter diagram was drawn according to the fluorescent intensity value of one cell. The apoptotic cell rate was calculated.
After t-test (SPSS10.0), sFasL in patients with HCC (2.8±0.4 pg/L) was different from that in patients with chronic hepatitis (3.2±0.4 pg/L), liver cirrhosis (3.8±1.1 pg/L) and normal control (3.5±0.7 pg/L) (P<0.05).
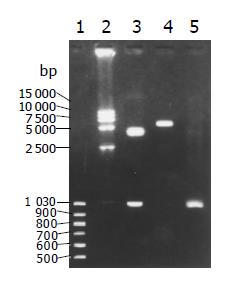
PCR and enzyme cut pCDNA31hisB-hFasL plasmid achieved the expected fragments (Figure 1).
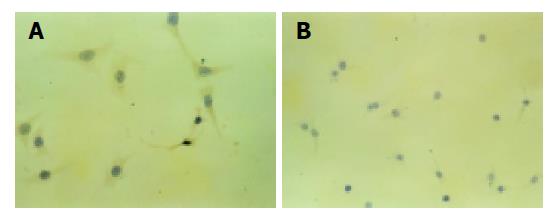
The concentrations of soluble FasL were 2029 and 2014 pg/L in supernatant of transfected HepG2 cells 24 and 48 h after they were cultured. No soluble FasL was detected in untransfected cells. The FasL was expressed on the membranes and cytoplasm of transfected cells. Control cells had no FasL expression. FasL positive expression appeared brown located on the membrane and cytoplasm, negative cells appeared blue, and both the cell nucleus appeared blue after being counterstained with sappan wood lignins (Figure 2).
The apoptotic cell rate was 36.30% in transfected cells and 11.53% in untransfected cells.
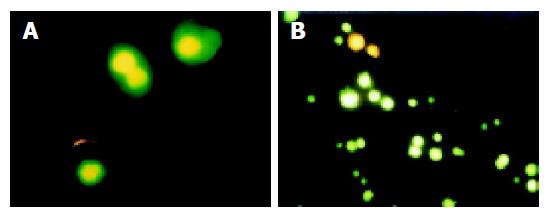
After being stained with annexin V and propidium iodine (PI), apoptotic cells could be distinguished under a microscope. The early apoptotic cells were stained by annexin V, but not stained by PI. The middle and late apoptotic cells were stained by both annexin V and PI. Dead cells were stained by PI and living cells were not stained by PI and annexin V (Figure 3).
Fas/FasL play an important role in HCC. Ito et al[14], evaluated Fas and Fas ligand expression in HCC patients, and found Fas and Fas ligand had a significant prognostic value for disease-free survival, and could be regarded as a new prognostic marker. Yoshitaka[15] found the labeling index of Fas/FasL was lower in cancerous liver tissue than in adjacent noncancerous region (P<0.01), and tended to decrease in proportion to the malignancy of tumor cells. Fas/FasL expression was not found in poorly-differentiated cancer cells. Fas(-)/FasL (+), FasL-mRNA (+) HCC cells were seen in one specimen of moderately-differentiated cells, suggesting that Fas/FasL expression was decreased in proportion to the malignancy of tumor cells, and infiltrating CD8+ T lymphocytes played a role in apoptosis in HCC. Also, the apoptosis in HCC could be regulated by the suppression of Fas/FasL expression, or sometimes, by the enhancement of FasL expression. Kupffer cells and LPS/Kupffer cell-conditioned supernatants might induce expression of Fas and Fas ligand in malignant glioma cells. Subsequently a significant proportion of malignant glioma cells became apoptotic, as evidenced by positive staining of annexin V and propidium iodine and an increase in cellular DNA fragmentation, suggesting a novel pathway of Kupffer cells against liver metastases, in which tumor cells were apoptotic via the Fas-Fas ligand system induced by TNF released from Kupffer cells. The suppression of Fas/FasL might have a correlation with HCC occurrence, malignancy and metastases[15-19].
Activated lymphocytes have been shown to release soluble forms of FasL (sFasL), which is capable of inducing apoptosis of Fas positive cells. sFasL is also known to induce activation of nuclear factor κB (NF-κB) and/or IL-8 secretion[20,21]. Thus, sFasL has biological activities, though the presence and possible function of sFasL in the liver have not been clarified yet. The present study, therefore, demonstrated that the amounts of sFasL in serum of patients with hepatocellular cancer had a significant difference compared with chronic viral hepatitis and liver cirrhosis, suggesting that sFasL plays an important role in HCC, and is worthy to be further studied.
In our study, soluble FasL was found in supernatant of transfected HepG2 cells and expressed on the membranes and cytoplasms of transfected cells, showing that Fas ligand was successfully transfected into HepG2 cells. The apoptotic rate in transfected HepG2 cells was higher than that in the control.
In conclusion, hepatoma cells become apoptotic via the CD95-CD95 ligand system without the involvement of immune cells. Fas L can induce apoptosis of hepatoma cells.
Co-first-authors: Yu-Huang Zheng
| 1. | Ciccocioppo R, Di Sabatino A, Parroni R, Muzi P, D'Alò S, Ventura T, Pistoia MA, Cifone MG, Corazza GR. Increased enterocyte apoptosis and Fas-Fas ligand system in celiac disease. Am J Clin Pathol. 2001;115:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Demir G, Ozguroglu M, Sayhan N, Molinas-Mandel N, Demirelli F, Buyukunal E, Tuzuner N, Serdengecti S, Berkarda B. Immunomodulating therapy with rIL-2 and interferon alpha-induces in vivo expression of Bcl-2, Fas (APO-1/CD95), and Fas ligand on peripheral lymphocytes (a pilot study). Anticancer Res. 1999;19:3517-3520. [PubMed] |
| 3. | Yoshino O, Matsuno H, Nakamura H, Yudoh K, Abe Y, Sawai T, Uzuki M, Yonehara S, Kimura T. The role of Fas-mediated apoptosis after traumatic spinal cord injury. Spine (Phila Pa 1976). 2004;29:1394-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Spielman J, Lee RK, Podack ER. Perforin/Fas-ligand double deficiency is associated with macrophage expansion and severe pancreatitis. J Immunol. 1998;161:7063-7070. [PubMed] |
| 6. | Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G629-G637. [PubMed] |
| 7. | Boulares AH, Zoltoski AJ, Stoica BA, Cuvillier O, Smulson ME. Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol. 2002;90:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Li B, Perabekam S, Liu G, Yin M, Song S, Larson A. Experimental and bioinformatics comparison of gene expression between T cells from TIL of liver cancer and T cells from UniGene. J Gastroenterol. 2002;37:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Abrahams VM, Straszewski SL, Kamsteeg M, Hanczaruk B, Schwartz PE, Rutherford TJ, Mor G. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 2003;63:5573-5581. [PubMed] |
| 10. | Rajashekhar G, Loganath A, Roy AC, Mongelli JM. Co-expression of Fas (APO-1, CD95)/Fas ligand by BeWo and NJG choriocarcinoma cell lines. Gynecol Oncol. 2003;91:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Siegel RM, Muppidi J, Roberts M, Porter M, Wu Z. Death receptor signaling and autoimmunity. Immunol Res. 2003;27:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | De Freitas I, Fernández-Somoza M, Essenfeld-Sekler E, Cardier JE. Serum levels of the apoptosis-associated molecules, tumor necrosis factor-alpha/tumor necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest. 2004;125:2238-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Oh SH, Yin HQ, Lee BH. Role of the Fas/Fas ligand death receptor pathway in ginseng saponin metabolite-induced apoptosis in HepG2 cells. Arch Pharm Res. 2004;27:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Ito Y, Monden M, Takeda T, Eguchi H, Umeshita K, Nagano H, Nakamori S, Dono K, Sakon M, Nakamura M. The status of Fas and Fas ligand expression can predict recurrence of hepatocellular carcinoma. Br J Cancer. 2000;82:1211-1217. [PubMed] |
| 15. | Fukuzawa K, Takahashi K, Furuta K, Tagaya T, Ishikawa T, Wada K, Omoto Y, Koji T, Kakumu S. Expression of fas/fas ligand (fasL) and its involvement in infiltrating lymphocytes in hepatocellular carcinoma (HCC). J Gastroenterol. 2001;36:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Lau WY, Chen GG, Lai PB, Chun YS, Leung BC, Chak EC, Lee JF, Chui AK. Induction of Fas and Fas ligand expression on malignant glioma cells by Kupffer cells, a potential pathway of antiliver metastases. J Surg Res. 2001;101:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Nakamoto Y, Kaneko S, Fan H, Momoi T, Tsutsui H, Nakanishi K, Kobayashi K, Suda T. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti-fas ligand antibody therapy. J Exp Med. 2002;196:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Mauz-Körholz C, Banning U, Körholz D. Regulation of interleukin-2 induced soluble Fas ligand release from human peripheral blood mononuclear cells. Immunol Invest. 2004;33:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Kaur H, Jaso-Friedmann L, Evans DL. Single base oligodeoxyguanosine upregulates Fas ligand release by nonspecific cytotoxic cells. Dev Comp Immunol. 2004;28:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Sakamoto N, Mukae H, Fujii T, Kakugawa T, Kaida H, Kadota J, Kohno S. Soluble form of Fas and Fas ligand in serum and bronchoalveolar lavage fluid of individuals infected with human T-lymphotropic virus type 1. Respir Med. 2004;98:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sugita S, Taguchi C, Takase H, Sagawa K, Sueda J, Fukushi K, Hikita N, Watanabe T, Itoh K, Mochizuki M. Soluble Fas ligand and soluble Fas in ocular fluid of patients with uveitis. Br J Ophthalmol. 2000;84:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |