Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2643
Revised: May 8, 2004
Accepted: June 24, 2004
Published online: May 7, 2005
AIM: To study the anti-hepatocarcinoma effects of 5-fluorouracil (5-Fu) encapsulated by galactosylceramide liposomes (5-Fu-GCL) in vivo and in vitro.
METHODS: Tumor-bearing animal model and HepA cell line were respectively adopted to evaluate the anti-tumor effects of 5-Fu-GCL in vivo and in vitro. Tumor cell growth inhibition effects of 5-Fu-GCL in vitro were assessed by cell viability assay and MTT assay. In vivo experiment, the inhibitory effects on tumor growth were evaluated by tumor inhibition rate and animal survival days. High performance liquid chromatography was used to detect the concentration-time course of 5-Fu-GCL in intracellular fluid in vitro and the distribution of 5-Fu-GCL in liver tumor tissues in vivo. Apoptosis and cell cycle of tumor cells were demonstrated by flow cytometry.
RESULTS: In vitro experiment, 5-Fu-GCL (6.25-100 μmol/L) and free 5-Fu significantly inhibited HepA cell growth. Furthermore, IC50 of 5-Fu-GCL (34.5 μmol/L) was lower than that of free 5-Fu (51.2 μmol/L). In vivo experiment, 5-Fu-GCL (20, 40, 80 mg/kg) significantly suppressed the tumor growth in HepA bearing mice model. Compared with free 5-Fu, the area under curve of 5-Fu-GCL in intracellular fluid increased 2.6 times. Similarly, the distribution of 5-Fu-GCL in liver tumor tissues was significantly higher than that of free 5-Fu. After being treated with 5-Fu-GCL, the apoptotic rate and the proportion of HepA cells in the S phase increased, while the proportion in the G0/G1 and G2/M phases decreased.
CONCLUSION: 5-Fu-GCL appears to have anti-hepato-carcinoma effects and its drug action is better than free 5-Fu. Its mechanism is partly related to increased drug concentrations in intracellular fluid and liver tumor tissues, enhanced tumor cell apoptotic rate and arrest of cell cycle in S phase.
- Citation: Jin Y, Li J, Rong LF, Li YH, Guo L, Xu SY. Anti-hepatocarcinoma effects of 5-fluorouracil encapsulated by galactosylceramide liposomes in vivo and in vitro. World J Gastroenterol 2005; 11(17): 2643-2646
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2643.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2643
5-Fluorouracil (5-Fu) is one of the broad-spectrum anti-tumor drugs[1-4], which has been confirmed to be the major therapeutic drug in liver carcinoma[5]. Despite many advantages, its clinical application is greatly limited due to its short half-life (T1/2Ke), wide distribution, low selectivity and various side effects[6-10]. Previous strategies were designed to overcome the following disadvantages such as in forms of microsphere, liposome, implants and nanoparticles[11-15]. In order to increase its hepatic selectivity and decrease its toxicity, galactosylceramide (GC) as a novel membrane material was adopted to envelope 5-Fu to form 5-Fu-GCL (Figure 1). The purpose of this study was to evaluate the anti-hepatocarcinoma effects of 5-Fu-GCL.
Cell line Mouse hepatocellular carcinoma cell line HepA, purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, was routinely maintained in RPMI1640 containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 U/mL streptomycin at 37 °C in a humidified atmosphere containing 50 mL/L CO2.
Animals Balb/c mice were purchased from the Experimental Animal Center of Anhui Medical University. The animals were maintained in a 12-h light/12-h dark cycle from 06:00 to 18:00 h under a regulated environment (20±1 °C). Animals were housed in plastic cages with free access to food and water. All experimental protocols described in this study were approved by the Ethics Review Committee for Animal Experimentation of Anhui Medical University.
Reagents 5-Fu galactosylceramide liposomes (batch no. 20020318, enveloped rate: 52%) were made at the Institute of Clinical Pharmacology, Anhui Medical University. 5-Fu (batch no. 20020318) was obtained from Jinan Pharmacy Corporation. Other chemicals used in experiments were of analytical grade from commercial sources.
Cell viability assay HepA cells were seeded on 24-well plates at a density of 5×104 cells/well for 24 h. On d 0, the medium was changed and then incubations were continued without or with increasing 5-Fu-GCL concentrations (0, 6.25, 12.5, 25, 50, 100 μmol/L) for 48 h. Dead cells were removed by gentle washing with PBS, and the number of living cells after treatment was counted using a hemocytometer by an experienced technologist who was unaware of the treatment conditions.
MTT assay Cells were seeded at a density of 5×104 cells/well in 96-well plates into HepA containing 10% FBS. After 24 h, fresh medium containing 5-Fu-GCL or free 5-Fu at concentrations (0, 6.25, 12.5, 25, 50, 100 μmol/L) was added for 48 h. Twenty microliters of stock MTT (5 mg/L) was added to each well. The absorbance was measured at a wavelength of 570 nm using an ELISA reader. The rate of cell growth inhibition was calculated by the formula: (the absorbance of control group-the absorbance of experimental group)/the absorbance of control group×100%.
Preparation of TB mice On d 0 following laparotomy, 107 HepA cells of approximately 0.1 mL of cell suspension were transplanted in the left lobe of the liver under slight ketamine anesthesia.
5-Fu-GCL treatment On d 4 after transplantation, tumor-bearing (TB) mice were randomly assigned to seven groups (20 mice/group) for treatment with 5-Fu-GCL at doses of 0, 20, 40, 80 mg/kg or free 5-Fu (40 mg/kg).
Tumor volume and survival days On d 18, 10 mice selected randomly from each group were killed, their solid tumors were excised and weighed. The rate of tumor inhibition was calculated by the formula: (the tumor volume of control group-the tumor volume of experimental group)/the tumor volume of control group×100%. The life span of other mice was investigated.
HPLC assay The concentration-time course of 5-Fu-GCL in intracellular liquor of HepA cells in vitro and the distribution of 5-Fu-GCL in liver tumor of TB mice in vivo were analyzed by high-performance liquid chromatography (HPLC). The mobile phase components were water and acetonitrile (99:1). The ultraviolet wavelength was 205 nm. The flow rate was 1 mL/min[16,17].
Flow cytometry Cells were treated with 5-Fu-GCL and free 5-Fu at various concentrations for 24 h. Cells were harvested with trypsin (2.5 g/L), washed with 0.01 mol/L PBS, fixed by cold alcohol at 4 °C, stained with propidium iodide (PI), and then analyzed by flow cytometry.
Statistical analysis of the data presented as mean±SD was performed using the Student’s t test. P<0.05 was considered statistically significant. IC50 was calculated by NDST program. Area under curve (AUC) was calculated by 3p97 program.
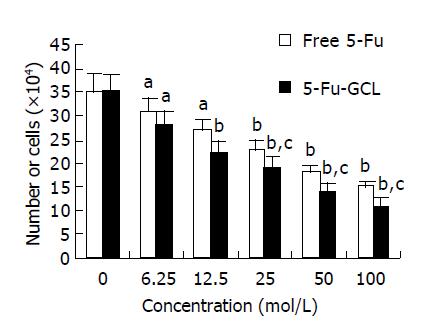
Cell counts The number of viable cells following incubation with either 5-Fu-GCL or free 5-Fu control medium for 48 h is shown in Figure 2. Cell growth was significantly inhibited after being treated with 5-Fu-GCL (6.25-100 μmol/L), and displayed a dose-dependent reduction of viable cells.
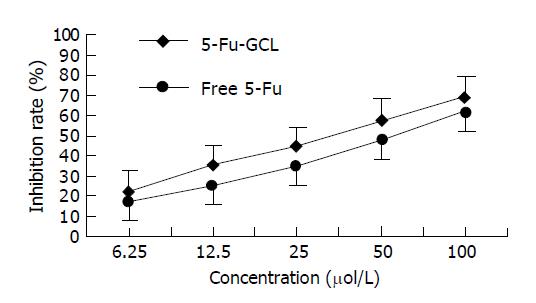
MTT assay In order to further investigate the growth inhibition of 5-Fu-GCL on HepA cells, cell growth was also determined by MTT assay. The rate of cell growth inhibition of 5-Fu-GCL is shown in Figure 3. The cell treated with 5-Fu-GCL displayed a dose-dependent inhibition. IC50 of 5-Fu-GCL (34.5 μmol/L) was lower than that of free 5-Fu (51.2 μmol/L).
The changes of tumor volume and survival time in TB mice treated with 5-Fu-GCL are shown in Table 1. One mouse in 5-Fu-GCL (80 mg/kg) group survived till the end of the experiment (50 d) and no viable tumor was found. Tumor volumes of TB mice treated with 5-Fu-GCL (20-80 mg/kg) were remarkably reduced. The survival time of TB mice treated with 5-Fu-GCL (40, 80 mg/kg) was remarkably prolonged.
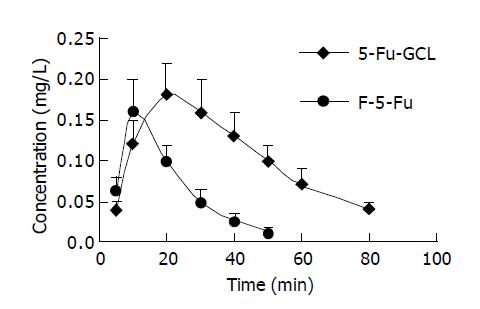
Concentration-time course of 5-Fu-GCL in intracellular fluid Data are shown in Figure 4. AUC of 5-Fu-GCL (21.5 mg∙min/L) in intracellular fluid of HepA cells at 50 μmol/L was 2.6 times higher than that of free 5-Fu (8.2 mg∙min/L).
Data are shown in Table 2. The concentration of 5-Fu-GCL was increased in a dose-dependent manner, and higher than that of free 5-Fu at 40 mg/kg.
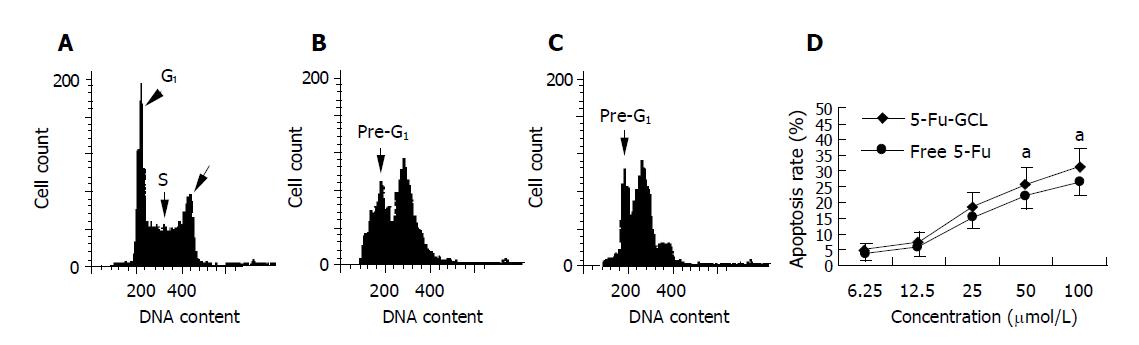
HepA cells were treated with various concentrations of 5-Fu-GCL for 48 h. DNA content of cells was measured by PI staining and flow cytometry analysis was made to detect apoptotic cells. The apoptotic cells could be observed on a DNA histogram as subdiploid or pre-G1 peak, the percentage of apoptotic cells treated with 5-Fu-GCL increased 4.5-30.7% of the total cell population (Figure 5).
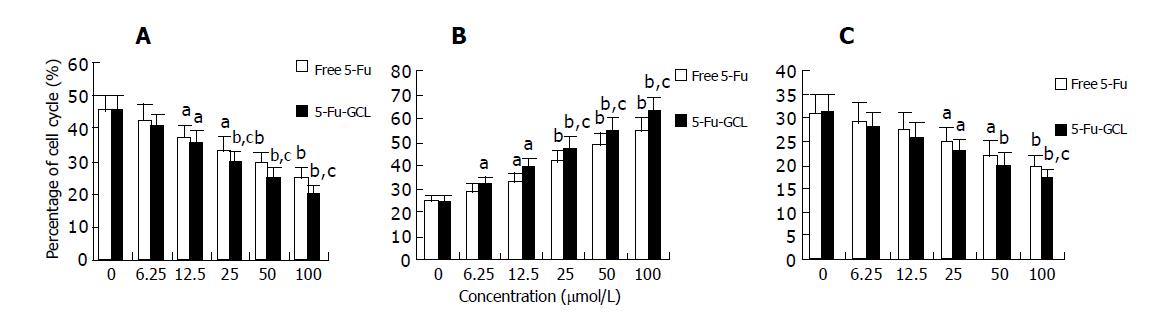
We assessed the effect of 5-Fu-GCL on cell cycle phase distribution of HepA cells using flow cytometry analysis. The HepA cells incubated for 48 h in control or 5-Fu-GCL or free 5-Fu medium are shown in Figure 6. The results showed that the proportion of HepA cells in the S phase increased, while the proportion in the G0/G1 and G2/M phases decreased.
As drug delivers[18-20], liposome could congregate drugs in certain tissues, decrease poisonous effects and increase the curative effects. Lecithoid material was the most primary liposome commonly used.
Since GC is a cerebroside containing galactose, liposome made of GC also possesses galactose. As a result, this kind of liposomes have a special binding ability to salivate acid protein receptors located on the membrane of mammals[21]. Compared with lecithoid, GC has several advantages such as stable chemical characteristics, long retention in blood, anti-oxide ability and unique directional trait. It may be developed into a new kind of medicine carriers.
5-Fu is a dissoluble chemical with a small molecule and confines it to be made into liposome with a higher enveloped rate[12]. In a recent study, we used calcium-induced fusion in combination with reverse evaporation to envelop Fu, and the enveloped rate reached 52%.
In our study, 5-Fu-GCL significantly inhibited not only the growth of HepA cells, but also tumor volume of TB mice. IC50 of 5-Fu-GCL was lower than that of free 5-Fu. The AUC of 5-Fu-GCL in intracellular fluid and the distribution of 5-Fu-GCL in liver tumor were significantly higher than those of free 5-Fu. 5-Fu-GCL induced apoptosis of HepA cells and increased the proportion of cells in the S phase of cell cycle and decreased the proportion of cells in the G0/G1 and G2/M phases of cell cycle, indicating that 5-Fu-GCL has anti-hepatocarcinoma effects, and the action of 5-Fu-GCL is stronger than that of free 5-Fu. It could partly increase drug concentrations in intracellular fluid and liver tumor, and enhance induction of apoptosis and cell cycle arrest in S phase.
In summary, the toxicity of 5-Fu-GCL is lower than that of free 5-Fu. 5-Fu-GCL can be developed into a novel preparation for liver cancer therapy.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Kubota T. Theoretical basis for low-dose CDDP/5-FU therapy. Gan To Kagaku Ryoho. 1999;26:1536-1541. [PubMed] |
| 2. | Benson AB. Therapy for advanced colorectal cancer. Semin Oncol. 1998;25:2-11. [PubMed] |
| 3. | Cao S, Rustum YM. Synergistic antitumor activity of irinotecan in combination with 5-fluorouracil in rats bearing advanced colorectal cancer: role of drug sequence and dose. Cancer Res. 2000;60:3717-3721. [PubMed] |
| 4. | Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, Hashimoto-Tamaoki T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61:1029-1037. [PubMed] |
| 5. | Aboagye EO, Saleem A, Cunningham VJ, Osman S, Price PM. Extraction of 5-fluorouracil by tumor and liver: a noninvasive positron emission tomography study of patients with gastrointestinal cancer. Cancer Res. 2001;61:4937-4941. [PubMed] |
| 6. | Kuropkat C, Griem K, Clark J, Rodriguez ER, Hutchinson J, Taylor SG. Severe cardiotoxicity during 5-fluorouracil chemotherapy: a case and literature report. Am J Clin Oncol. 1999;22:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Fata F, Ron IG, Kemeny N, O'Reilly E, Klimstra D, Kelsen DP. 5-fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer. 1999;86:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | van Kuilenburg AB, Haasjes J, Richel DJ, Zoetekouw L, Van Lenthe H, De Abreu RA, Maring JG, Vreken P, van Gennip AH. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705-4712. [PubMed] |
| 9. | Di Paolo A, Danesi R, Falcone A, Cionini L, Vannozzi F, Masi G, Allegrini G, Mini E, Bocci G, Conte PF. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol. 2001;12:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Kuan HY, Smith DE, Ensminger WD, Knol JA, DeRemer SJ, Yang Z, Stetson PL. Regional pharmacokinetics of 5-fluorouracil in dogs: role of the liver, gastrointestinal tract, and lungs. Cancer Res. 1998;58:1688-1694. [PubMed] |
| 11. | Martini LG, Collett JH, Attwood D. The release of 5-fluorouracil from microspheres of poly(epsilon-caprolactone-co-ethylene oxide). Drug Dev Ind Pharm. 2000;26:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Hagiwara A, Sakakura C, Shirasu M, Yamasaki J, Togawa T, Takahashi T, Muranishi S, Hyon S, Ikada Y. Therapeutic effects of 5-fluorouracil microspheres on peritoneal carcinomatosis induced by Colon 26 or B-16 melanoma in mice. Anticancer Drugs. 1998;9:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Jing M, Xi S, Chen R. The inhibitory effect of tissue plasminogen activator combined with 5-fluorouracil polyphase liposome on the scar formation in experimental filtration surgery. Zhonghua YanKe ZaZhi. 1997;33:376-380. [PubMed] |
| 14. | Wang G, Tucker IG, Roberts MS, Hirst LW. In vitro and in vivo evaluation in rabbits of a controlled release 5-fluorouracil subconjunctival implant based on poly(D,L-lactide-co-glycolide). Pharm Res. 1996;13:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Umejima H, Kikuchi A, Kim NS, Uchida T, Goto S. Preparation and evaluation of Eudragit gels. VIII. Rectal absorption of 5-fluorouracil from Eudispert hv gels in rats. J Pharm Sci. 1995;84:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Jung M, Berger G, Pohlen U, Päuser S, Reszka R, Buhr HJ. Simultaneous determination of 5-fluorouracil and its active metabolites in serum and tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;702:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Joulia JM, Pinguet F, Grosse PY, Astre C, Bressolle F. Determination of 5-fluorouracil and its main metabolites in plasma by high-performance liquid chromatography: application to a pharmacokinetic study. J Chromatogr B Biomed Sci Appl. 1997;692:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 406] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Kim S. Liposomes as carriers of cancer chemotherapy. Current status and future prospects. Drugs. 1993;46:618-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kaneda Y. Virosomes: evolution of the liposome as a targeted drug delivery system. Adv Drug Deliv Rev. 2000;43:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Nishikawa M, Hirabayashi H, Takakura Y, Hashida M. Design for cell-specific targeting of proteins utilizing sugar-recognition mechanism: effect of molecular weight of proteins on targeting efficiency. Pharm Res. 1995;12:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |