Published online Apr 21, 2005. doi: 10.3748/wjg.v11.i15.2255
Revised: March 31, 2004
Accepted: May 24, 2004
Published online: April 21, 2005
AIM: To investigate the effects and mechanisms of Verapamil on cultured human colonic tumor (HCT) cells.
METHODS: HCT cells were treated with different concentrations of Verapamil, and their proliferation was examined by MTT assay. The areas of sub-diploid peak were measured by flow cytometry, and the DNA ladder was found by agarose gel electrophoresis. The characteristic changes in morphology were observed under light microscopy. The cell nuclei (propidium iodide labeled, PI-labeled) and cellular distribution and concentration of calcium (Fluo-3-labeled) were studied by using laser confocal scanning microscope.
RESULTS: The proliferation of HCT cells was inhibited by different concentrations of Verapamil. With the increase in concentration of Verapamil, the percent of G0-G1 phase cells in HCT cells increased and that of S phase cells decreased. After treating with different concentrations of Verapamil, flow cytometry showed that HCT cells were enlarged in areas of sub-diploid in a dose-dependent manner. Gel electrophoresis results displayed a typical DNA ladder. On staining with Wrights-Giemsa, the typical cellular apoptosis morphologic changes were also observed. PI-labeled cell nuclei were found markedly changed. In addition, we inspected that the 100 μmol/L Verapamil could increase the intracellular calcium ion concentration [Ca2+]i in HCT cells.
CONCLUSION: Verapamil can inhibit proliferation of HCT cells via inducing cell apoptosis.
-
Citation: Cao QZ, Niu G, Tan HR.
In vitro growth inhibition of human colonic tumor cells by Verapamil. World J Gastroenterol 2005; 11(15): 2255-2259 - URL: https://www.wjgnet.com/1007-9327/full/v11/i15/2255.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i15.2255
Due to the critical role of calcium in vital activities, the studies about the impacts of calcium antagonists on the diseases are of practical significance. In the USA, there are about seven million people who use calcium antagonists to treat cardiovascular or cerebrovascular diseases. The uses of these drugs are always considered to have good therapeutic effect[1,2].
Many researchers have demonstrated that the increase of intracellular calcium concentration played an important role in cell apoptosis[3], and the apoptosis of cells is related to the growth of tumor cells[4]. So, it is particularly important to know whether the potential risk of increasing the growth of tumors will exist after the patient takes calcium antagonists orally for a long time. In this study, a series of experiments were carried out aiming at investigating the influence of Verapamil on the tumor cells. Our study demonstrated that the calcium antagonist, Verapamil, might inhibit the proliferation of HCT cells through inducing apoptosis.
HCT cell line was a generous gift from Professor Cui Jin-Rong (Natural and Imitative Drug Important Laboratory). Verapamil was obtained from Henrini Medical Company in Jiangshu Province, China. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was purchased from Amresco (Amresco, USA) Company. PI (Propidium iodide) was purchased from Sigma (Sigma, S. Louis, MO, USA). Trypsin was purchased from Gibco (Gibco BRL, USA).
HCT cells were routinely cultured with RPMI 1640 medium supplemented with 100 mL/L fetal bovine serum at 37 °C in a humidified atmosphere containing 50 mL/L CO2.
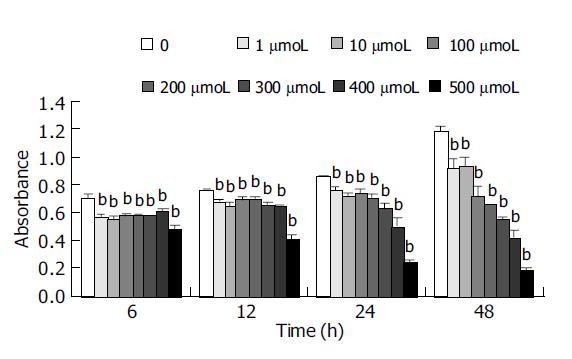
Cells were dissociated with 2.5 g/L trypsin and resuspended in tissue culture media to yield a concentration of 1×105 cells/mL. Cells were added to 96-well plates (9000 cells/well), and allowed to attach for 24 h at 37 °C. Verapamil was added at the concentration 1, 10, 100, 200, 300, 400, 500 μmol/L (10 μL/well), respectively. Control wells contained only culture medium. After the cells were incubated with these additives for 6, 12, 24 and 48 h cell proliferation was estimated based on the cellular reduction of tetrazolium salt MTT[5] by using a microplate reader (BIO-RAD, Model 550 USA), at a test wavelength of 540 nm.
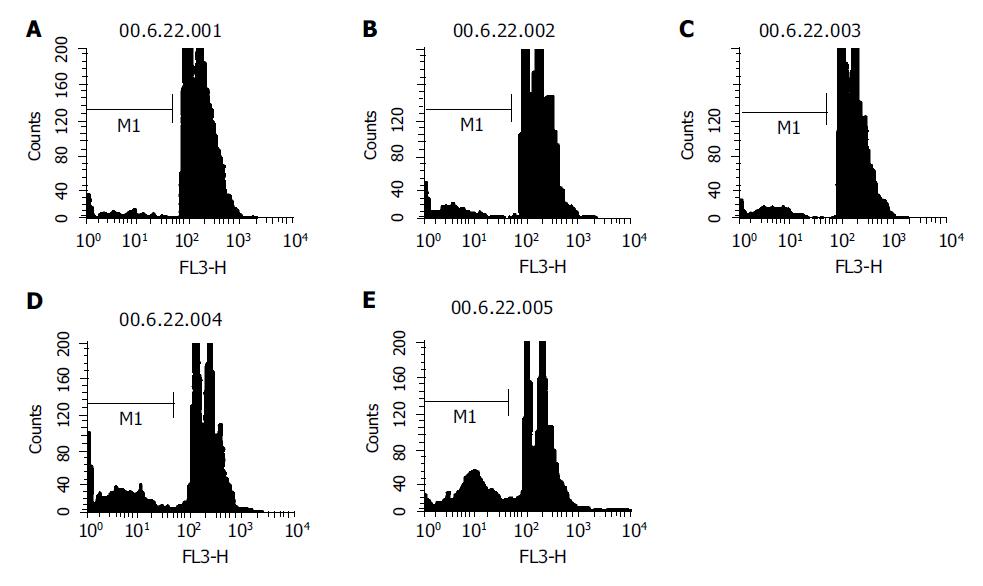
The HCT cells were incubated with different concentrations of Verapamil (1, 10, 100 and 500 μmol/L) for 48 h, fixated in 700 mL/L cold ethanol (-20 °C) after washing twice with PBS (phosphate buffered saline, without Ca2+ or Mg2+), and stocked at 4 °C overnight. The cells were washed twice with PBS and the cell concentration was adjusted to 1×106/mL, and 1 mL of cell suspension was added with 10 μL of 10 g/L RNase A and incubated at 37 °C for 30 min. Then, PI was added into the cells at a concentration of 50 mg/L. Cell apoptosis and cell cycle was analyzed with flow cytometry.
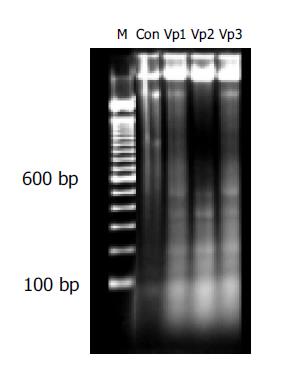
DNA was isolated from cells according to routine protocol[6]. Briefly HCT cells treated with or without Verapamil (100, 200 and 400 μmol/L) were harvested after 48 h incubation. Cells were centrifuged at 800 r/min and washed with PBS. After cells were resuspended at a concentration of 1×106 cells/mL in extraction buffer (10 mmol/L Tris-HCl, 0.1 mol/L EDTA, 5 g/mL SDS), they were treated with 20 mg/L RNase A at 37 °C for 60 min, followed by incubation with 100 mg/L proteinase K at 37 °C for 60 min. Equal volume of phenol: chloroform: isopropyl alcohol (25:24:1) was added to the cells and centrifuged at 13000 g for 10 min. The liquor of supernatant was collected, and then 0.1 volume NaAc (3 mol/L pH 5.2) and 2 volume ethanol (-20 °C) were added, and stocked at -20 °C overnight. The samples were centrifuged at 13000 g for 30 min at 4 °C. Then the liquor of supernatant was discarded and the pellets were dissolved in 1×TE buffer. The concentration of DNA was detected by UV spectrophotometer (Beckman DU-640, USA). The DNA content (5 μg/tube) was transferred to the 20 g/L agarose gel, horizontal gel electrophoresis was performed at 60 V for 90 min and the DNA in gels was visualized under gel image analysis (BIO-RAD, Gel DOC 2000, USA) after staining with 5 μg/mL ethidium bromide.
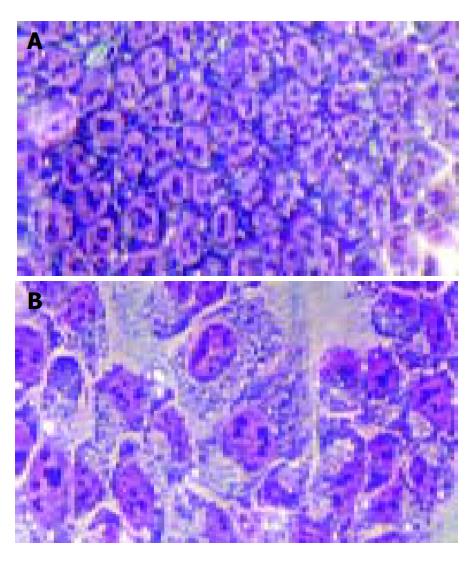
In the routine staining with Wrights-Giemsa, the HCT cells were incubated with 100 μmol/L Verapamil for 48 h, the liquor of supernatant was discarded, and cells were fixated and air-dried after washing twice with PBS, and stained with Wrights for 4 min, then with Giemsa for 6 min. Dyestuff was discarded and cells were washed. Then, the cell morphology was observed under microscope.
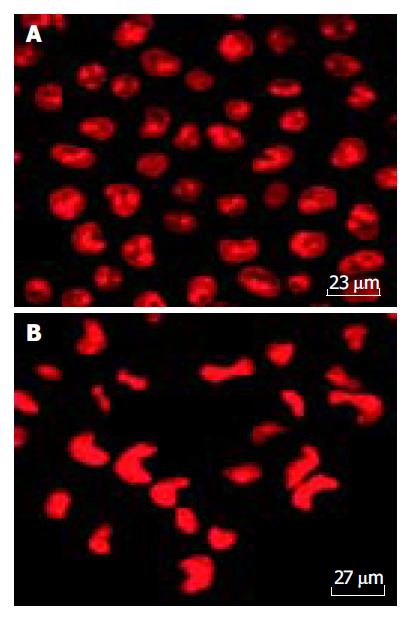
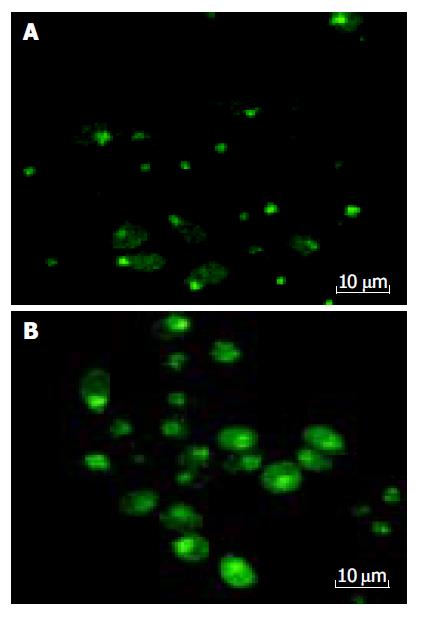
Cells were dissociated with 2.5 g/L trypsin and resuspended in tissue culture media to yield a concentration of 1×105 cells/mL. Cells were allowed to attach to Petri dish for 24 h at 37 °C a humidified 50 mL/L CO2/950 mL/L air incubator. Verapamil was added at a concentration of 100 μmol/L. An equal volume of PBS was added to the control instead. The cells were incubated with these additives for 12 h. Then the liquor of supernatant was discarded, the cells were treated with Fluo-3/AM at a concentration of 5 μmol/L for marking [Ca2+]i, and PI (50 mg/L) for nuclear (Editor query: is something missing here?) after washing twice with PBS, the cellular concentration of calcium and nuclear shape diversification were analyzed using the confocal scanning laser microscopy (TCS-NT, Leica).
All results are expressed as mean±SD. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 11.0 for Windows.
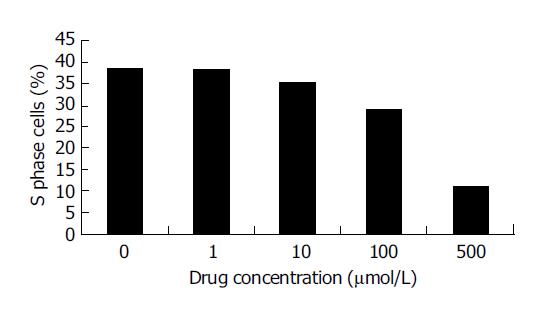
After HCT cells were incubated with different concentrations of Verapamil for 6, 12, 24 or 48 h, we observed a significant inhibition in proliferation of HCT cells in a dose-dependent manner by MTT assay (Figure 1). With the increase in concentration of Verapamil, the percent of G0-G1 phase cells in HCT cells was increased and S phase cells were decreased as detected by flow cytometry. Cell cycle analysis showed that the proliferation of HCT cells was blocked at G0-G1 phase (Figure 2).
HCT cells were treated with different concentrations of Verapamil, and the data from flow cytometry showed the areas of sub-diploid peak that were enlarged in drug-treated HCT cells, suggesting that the percentage of apoptosis cells increased as Verapamil concentration increased (Figure 3).
After HCT cells were treated with different concentrations of Verapamil, a typical DNA ladder was observed, which suggested the induction of apoptosis in Verapamil-treated HCT cells (Figure 4).
During routine staining with Wrights-Giemsa, the typical cellular morphologic changes, including cell membrane blebs, and the cytoplasm and nuclear chromatin condensation, were observed (Figure 5). The cell nuclei were marked with fluorescence-probe PI and found markedly changed, most of the cell nuclei were present in reniform and nuclear chromatin condensation (Figure 6).
The [Ca2+]i was determined with the laser confocal scanning microscopy, which demonstrated that 100 μmol/L Verapamil could increase the concentration of [Ca2+]i in HCT cells (Figure 7 and Table 1).
| Groups | [Ca2+]i intensity |
| Control | 28.37±11.94 |
| Verapamil (100 mmol/L) | 43.34±11.58b |
A series of experiments have demonstrated that the apoptosis of cells is induced by radiation or drugs and the concentration of free intracellular Ca2+ is found to be markedly elevated in the apoptotic cells[7-12]. Intracellular Ca2+ is the key-initiating factor during the course of apoptosis of cell line[13]. Now calcium channel antagonists are widely used clinically to treat cardiovascular and cerebrovascular diseases; the routine dosage of these drugs taken orally can improve the symptoms of cardiovascular and cerebral-vascular diseases[14,15]. Do they produce a certain influence on the patients with these diseases complicated by tumors? Are they related to the danger of carcinogenesis and cancer development? And what is the mechanism of their influences on tumors? All of these problems are still of great concern and need to be solved by epidemiologists, clinicians and researchers of basic sciences.
Our study of the effect of Verapamil on cell proliferation demonstrated that Verapamil might inhibit the growth of HCT cells. Results from flow cytometry showed that HCT cells after treating with calcium antagonist, the hypodiploid peak area was increased significantly as the concentrations of Verapamil were increased, which indicated that the proportion of apoptotic cells increased. This result was also proved by cellular morphologic changes, such as cell membrane blebs and the cytoplasm and nuclear chromatin condensation. At the same time, the karyotype of PI-labeled cells was found markedly changed and most of the cell nuclei were present in reniform as detected by confocal scanning microscopy. Our above findings were quite enough to show that the calcium antagonist markedly increased cell apoptosis. Jensen et al[16-18] also demonstrated that calcium channel antagonists could inhibit meningioma growth in vitro and via interfering with intracellular signaling pathways of cultured meningioma cells. Jensen and Wurster[19] also showed that calcium channel antagonists could decrease but not completely inhibit xenograft meningioma growth over time compared to control groups in nude mice. In this study, flow cytometry showed that as the concentration of Verapamil increased, the percentage of S-phase cells in the whole cycle was lowered, whereas the proportion of G0/G1-phase cells increased, which indicated that Verapamil could block the transformation of HCT cells from G0/G1-phase into S-phase, and interfere with the process of DNA replication in these cells. The proliferation of HCT cells was also inhibited by blocking cell cycle after the use of the calcium antagonist.
In our experiment, when HCT cells were treated with 100 μmol/L Verapamil for 12 h, the intracellular calcium ion concentration [Ca2+]i detected by Fluo-3AM fluorescence-labeling method, was not surprisingly increased. Why does the intracellular concentration of calcium ions within HCT cells rise after the use of calcium antagonists? Usually, scientists consider that after the use of calcium antagonists, as the entry of extracellular calcium ions is inhibited, the intracellular concentration of calcium ions can then be kept at a relatively low level; but in this experiment we obtained a surprising result. How did the calcium antagonist raise the concentration of calcium ions within the HCT cells? What was the mechanism for that? Did the drug have the tendency to produce only a single effect of preventing the outflow of calcium ions? These problems need to be further investigated. The more easily accepted theory is that the use of calcium antagonists may destroy the mobile equilibrium between intracellular and extracellular calcium ions, which causes the calcium ions that are stored in the cell organs to be released into the cytoplasm, resulting in the increase of the intracellular concentration of calcium ions.
In conclusion, the use of the calcium antagonist, Verapamil, produces a growth inhibiting effect on HCT cells by increasing the apoptosis of HCT cells and also by blocking cell cycle. The problem whether calcium antagonists can take part in or contribute to the treatment for tumors is to be further studied, and researches should be made through many new relevant subjects.
We thank the members of our laboratory for the stimulating discussions.
Science Editor Kumar M Language Editor Elsevier HK
| 1. | Mason RP. Effects of calcium channel blockers on cellular apoptosis: implications for carcinogenic potential. Cancer. 1999;85:2093-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Qing SH, Rao KY, Jiang HY, Wexner SD. Racial differences in the anatomical distribution of colorectal cancer: a study of differences between American and Chinese patients. World J Gastroenterol. 2003;9:721-725. [PubMed] |
| 3. | Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1418] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 4. | Wright SC, Zhong J, Zheng H, Larrick JW. Nicotine inhibition of apoptosis suggests a role in tumor promotion. FASEB J. 1993;7:1045-1051. [PubMed] |
| 5. | Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936-942. [PubMed] |
| 6. | Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: A laboratory manual, 1st edition New York, USA, Cold Spring Haobor Laboratory Press 1982: 280-281. . |
| 7. | Macho A, Blanco-Molina M, Spagliardi P, Appendino G, Bremner P, Heinrich M, Fiebich BL, Muñoz E. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem Pharmacol. 2004;68:875-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Leger DY, Liagre B, Corbière C, Cook-Moreau J, Beneytout JL. Diosgenin induces cell cycle arrest and apoptosis in HEL cells with increase in intracellular calcium level, activation of cPLA2 and COX-2 overexpression. Int J Oncol. 2004;25:555-562. [PubMed] |
| 9. | Groenendyk J, Lynch J, Michalak M. Calreticulin, Ca2+, and calcineurin - signaling from the endoplasmic reticulum. Mol Cells. 2004;17:383-389. [PubMed] |
| 10. | Sharma AK, Rohrer B. Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem. 2004;279:35564-35572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 394] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Lemarié A, Lagadic-Gossmann D, Morzadec C, Allain N, Fardel O, Vernhet L. Cadmium induces caspase-independent apoptosis in liver Hep3B cells: role for calcium in signaling oxidative stress-related impairment of mitochondria and relocation of endonuclease G and apoptosis-inducing factor. Free Radic Biol Med. 2004;36:1517-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 391] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Xu RX, Tu YY, Zou CY, Yang ZL, Chen YZ, Cai YQ, Du MX. Reinforcement of Nimodipine to the TK Suicide Gene Therapy of Glioma. China J Cancer Prev Treat. 2003;10:1129-1133. |
| 15. | Li Y, Wu JZ, Liu B, Duan XH, Li F, Li J. Synergic anti-tumor effect of combination of verapamil with chemotherapy on highly metastatic human mucoepidermoid carcinoma(Mc3) cells. J Fourth Mil Med Univ. 2003;24:2259-2261. |
| 16. | Jensen RL, Origitano TC, Lee YS, Weber M, Wurster RD. In vitro growth inhibition of growth factor-stimulated meningioma cells by calcium channel antagonists. Neurosurgery. 1995;36:365-373; discussion 373-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Jensen RL, Lee YS, Guijrati M, Origitano TC, Wurster RD, Reichman OH. Inhibition of in vitro meningioma proliferation after growth factor stimulation by calcium channel antagonists: Part II--Additional growth factors, growth factor receptor immunohistochemistry, and intracellular calcium measurements. Neurosurgery. 1995;37:937-946; discussion 946-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Jensen RL, Petr M, Wurster RD. Calcium channel antagonist effect on in vitro meningioma signal transduction pathways after growth factor stimulation. Neurosurgery. 2000;46:692-702; discussion 702-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Jensen RL, Wurster RD. Calcium channel antagonists inhibit growth of subcutaneous xenograft meningiomas in nude mice. Surg Neurol. 2001;55:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |