Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2130
Revised: February 28, 2004
Accepted: April 20, 2004
Published online: April 14, 2005
AIM: To investigate the combined effect of STI571 and p27 gene clone on the regulation of proliferation, cell cycle and apoptosis of K562 cell line.
METHODS: p27 gene was obtained by RT-PCR, and its sequence was approved to be correct. Then p27-pcDNA3.1 vector was constructed and transfected into K562 cell line. p27-pcDNA3.1-K562 cell clone was screened by G418 after transfection, p27 protein was identified by Western blot. MTT was used to detect the survival rate of the cell. Flow cytometry was used to detect cell cycle and apoptosis index.
RESULTS: The expression of p27 protein could be detected by Western blot in p27-pcDNA3.1-K562 cells. A strong inhibition of cell proliferation was observed in p27-pcDNA3.1 -K562 cells as compared with that of the control (pcDNA3.1 -K562 cells). The cells at G0/G1 phase were significantly increased, and cells at S phase were greatly declined. The apoptosis index was increased greatly after p27-pcDNA3.1-K562 cells were treated with STI571, and survival rate of the cell was markedly declined (0.35-0.58, P<0.05-0.048 vs STI571-K562 cell, 0.35-0.72, P<0.01-0.001 vs p27-K562 cell).
CONCLUSION: p27 and STI571 have a synergistic action on inhibition of proliferation and induction of apoptosis on K562 cells.
- Citation: Wang W, Yao LB, Liu XP, Feng Q, Shang ZC, Cao YX, Sun BZ. Effects of STI571 and p27 gene clone on proliferation and apoptosis of K562 cells. World J Gastroenterol 2005; 11(14): 2130-2135
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2130.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2130
STI571, a tyrosine kinase inhibitor used in clinic, can inhibit the tyrosine kinase of P210 protein encoded by BCR-ABL (Breakpoint cluster region and Abelson leukemia virus oncogene) fusion gene with a high selectivity[1,2]. Only the tyrosine kinase of platelet-derived growth factor[3,4] is inhibited at a similarly low concentration. This activity is believed essential for malignant transformation[5]. So it has an optimal therapeutic action on K562 cell line and sets up an epoch era in molecular targeted therapy. But multidrug resistance, recurrence and poor curative effect to acute phase cells were its major problems[6-8]. So combined therapy should be a new direction for future therapeutic development. p27 is an important member of the cyclin-dependent kinase inhibitor family and has been reported as a tumor suppressor gene[9,10], and its deletion, mutation or down-regulation play an important role in tumorigenesis[11,12]. So combined treatment of p27 and STI571 may exert an obvious therapeutic effect on K562 cells.
STI571 was kindly provided by Novartis Company (Basel, Switzerland). A 1.0 mmol/L stock solution of STI571 in distilled water was prepared, filtrated and sterilized. pcDNA3.1 eukaryote expression plasmid was purchased from Invitrogen Company. E.coli JM109 cell strain was kindly provided by Biochemistry and Molecular Biology Laboratory of The Fourth Military Medical University (Xi’an, China). Digestion enzymes EcoRI and HindIII, Taq DNA, reverse transcriptase, and T4 ligase were purchased from Santa Cruz or Sangon Company. TRIzol RNA reagent and DNA purifying reagent were from Invitrogen Company. Mouse monoclonal antibody against p27 and goat anti-mouse antibody were purchased from Santa Cruz Company.
K562 cells (containing BCR-ABL fusion gene, expressing P210 protein[13]) were grown in RPMI1640 medium supplemented with 10 mL/L fetal calf serum, penicillin, streptomycin, and L-glutamine, at 37 °C in an incubator containing 50 mL/L CO2.
Upstream primer (5’GTAAGCTTATGTCAAACGT-GCGAGTGTCTA3’) and downstream primer (5’TGGAA-TTCTTACGTTTGACGTCTTCTGAGG3’) were used to amplify p27 gene. The sequences of upstream and downstream primers contained restriction sites of HindIII and EcoRI (restriction endonuclease) respectively in order to digest PCR products or vectors. The PCR product of these primers was about 600 bp in length.
Total RNA was extracted from peripheral blood mononuclear cells (PMC) with TRIzol reagent following the manufacturer’s instructions. Then 2.0 μg of total RNA was reverse transcribed with M-Mulv reverse transcriptase and oligo(dT). PCR reaction mixture (25 μL) containing 0.2 μg of cDNA, 0.5 μL of 10 mmol/L dNTP, 10 pmoL of each primer and 1 U Taq polymerase was subjected to 30 amplification cycles, each cycle consisting of denaturation at 94 °C for 45 s, annealing at 55 °C for 40 s, and extension at 72 °C for 1 min. An additional extension at 72 °C for 30 min was performed. PCR products were electrophoresed on 1% agarose gels and stained with ethidium bromide (EB). Photographs of EB-stained gels were taken.
Total RNA was extracted from PMC and K562 cells respectively, and reverse transcribed into cDNA, and quantified by absorbance at 260 nm, then 20 μg of cDNA was electrophoresed on 0.8% alkalescence agarose gels. DNA was transferred to Hybond-N membranes and subjected to Southern blotting. DNA was fixed in Hybond-N membranes by ultraviolet irradiation. Hybridized membranes were wetted with 6×SSC, and then pre-hybridized for 3 h at 68 °C. Then denaturalized probes were joined and hybridized with DNA overnight at 68 °C. Hybridized membranes were washed with 2×SSC/0.1% SDS and 0.2×SSC/0.1% SDS twice and reserved at -70 °C for 5-7 d. p27 was detected by hybridization with its respective full-length 32P-labeled PMC cDNA probes and imaged by autoradiography[14].
After PCR products were separated by electrophoresis, positive agarose gels were sliced, and then PCR products were purified and call backed using a DNA purifying kit, and deposited by ethanol.
Reaction mixture contained 5 μL of purified DNA, 1 μL of T vector, and 4 μL of rapid ligation fluid. After being incubated at 16 °C for 4 h, the mixture was transduced into JM109 cell strain. The transduced cells were detected by staining with 5-bromo-4 chloro-3 indolyl β-D-galactopyranosides (X-gal). A white positive clone was chosen and sent to Sagon Company to test its sequence.
pcDNA3.1 and T-p27 vectors were digested with HindIII and EcoRI. Then pcDNA plasmid and p27 were ligated with T4 ligase. After pcDNA-p27 vector was transduced into JM109 strain, the positive clones were used to pick-up plasmids, which were transfected into K562 cells with lipofectine as previously described. After 48 h, the cells were cultured in RPMI1640 medium containing G418 (500 mL/L) for 28 d to select the stably transfected positive clones[15] (pcDNA3.1-p27-K562 cell clone).
Western blot extracts were prepared by lysing K562 cells with lysis buffer[16] (20 mmol/L HEPES, pH 7.9, 150 mmol/L NaCl, 1.0 mmol/L MgCl2, 5 mmol/L EDTA, pH 8, 0.1% nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L NaF, 5 mmol/L sodium orthovanadate) on ice for 20 min. Fifty micrograms of lysate was separated by SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and specific proteins were recognized by mouse moloclonal antibody against p27 and cyclin-E. Membranes were incubated for 1 h at room temperature with mouse moloclonal antibody against p27 and cyclin-E. After three washes in TBS-T (Tris 10 mmol/L, NaCl 50 mmol/L, Tween 0.005%, pH 8.0), membranes were incubated for 1 h at room temperature with goat anti-mouse IgG horseradish peroxidase-linked at 1/3000 final dilution. After three washes in TBS-T, membranes were revealed with the enhanced chemiluminescent detection system[17] (Pierce Biotech).
Cells (5×104/L) were put in each well of a 96-well plate, and divided into p27 group, STI571 group, p27 and STI571 group. Each group had six different concentrations of cells, and each concentration of cells was put in three wells to calculate the average value. The cells were cultured for 5 d and the cells were calculated everyday to determine the growth inhibition and survival rate of the cells.
After p27-pcDNA vector was transfected into K562 cells, the cells were treated with STI571. After 48 h, the cells were collected by centrifugation, washed twice with phosphate-buffered saline, and permeabilized in 70 mL/L ethanol (-20 °C). The permeabilized cells were incubated with 50 μg/mL of propidium iodide, 0.1 μg/mL of Rnase A, 0.1% Nonidet P-40, and 50 μg/mL trisodium citrate for 30 min prior to analysis using a Becton Dickinson FACSort analyzer.
One-way ANOVA was used to compare the data between groups, and Dunnett’s t test was used to compare the data within each group by using SPSS11.0 statistic software.
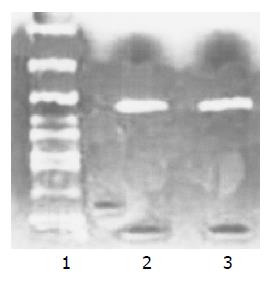
The complete cDNA sequence of p27 gene was amplified from PMC by RT-PCR to construct p27-pcDNA3.1 recombinant vector, and RT-PCR products were electro-phoresed in 1% agarose gels and stained with EB. The amplified DNA fragments (about 600 bp) were completely consistent with p27 gene full-length cDNA. Meanwhile a DNA fragment (about 600 bp) was amplified from K562 cells using the same primers (Figure 1).
p27 gene deletion in K562 cells was proved by Southern blot (Figure 2).
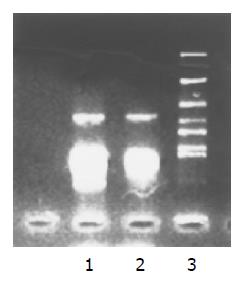
The complete cDNA sequence of p27 gene amplified from PMC and pcDNA3.1 vector was digested with HindIII and EcoRI, and ligated with T4 ligase, and the recombinant p27-pcDNA3.1 vector was constructed. The recombinant vector was digested with HindIII and EcoRI. The size of digested DNA fragments was completely consistent with p27 gene full-length cDNA (Figure 3).
p27 gene amplified from PMC was ligated with PUC18-T vector to test p27 gene sequence, and the result proved that its sequence was correct with the designed p27 gene sequence (Figure 4A). Meanwhile the tested sequence of DNA fragments amplified from K562 cells was absolutely incorrect with p27 gene (Figure 4B). That means it was a non-specific amplification. Thus p27 gene deletion in K562 cells was proved.
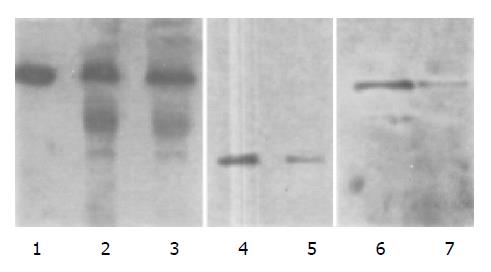
After p27-pcDNA3.1 recombinant vector was stably transfected into K562 cells by using lipofectine for 2 wk, a cell line stably expressing p27 protein was established. In this cell line, p27 protein expression was proved by Western blot, whereas cyclin-E protein expression was decreased. Protein quantity analysis was performed by Lowry’s method, and β-actin was used as an internal control (Figure 5).
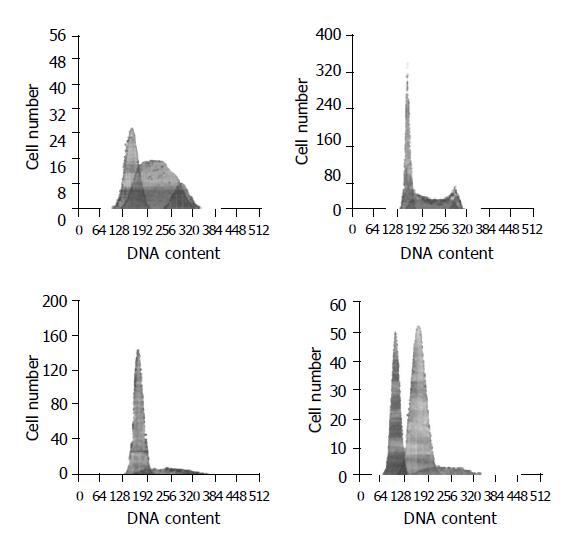
The number of S phase cells was increased in the control K562 cells. The percentage of cells was 30.2% in G1 phase, 57.4% in S phase and 12.3% in G2/M phase. After p27-pcDNA3.1 recombinant vector was transfected into K562 cells, the percentage of S phase cells was significantly decreased, while the percentage of G1 phase cells was increased apparently, indicating that p27 gene could inhibit K562 cell proliferation and decrease S phase cells by cell cycle arresting in G1 phase. When K562 cells were treated with STI571 for 48 h (>0.5 μmol/L), apoptosis apex could be observed in flow cytometry map, and it increased with the increase of STI571 concentration. STI571 could induce apoptosis in a dose-dependent manner. In control K562 cells, apoptosis apex was not observed when the concentration of STI571 was 0.25 μmol/L. But in K562-p27-pcDNA3.1 cells, apoptosis apex (32.4%) was observed when treated with the same concentration of STI571 (0.25 μmol/L) for 48 h, and the percentage of S phase cells was decreased apparently, suggesting the synergistic action of p27 and STI571 on induction of apoptosis and inhibition of proliferation of K562 cells (Figure 6).
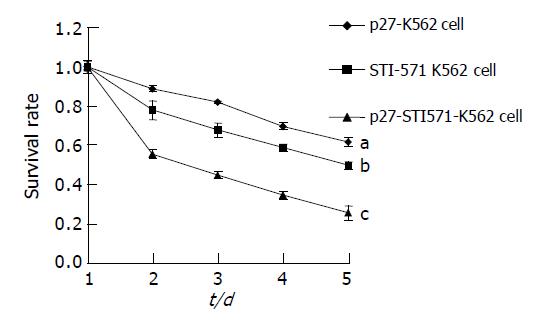
MTT assay showed that K562 cells proliferated rapidly and the population doubling time was about 18-24 h. Little change was found in growth rate when control pcDNA3.1 vector was transfected into K562 cells, but the growth rate of K562 cells transfected with p27-pcDNA3.1 was apparently slower than that of the control K562 cells, and the population doubling time was about 48-60 h, indicating that p27 protein could inhibit K562 cell proliferation. STI571 could also apparently inhibit K562 cell proliferation, and the cell survival rate declined in a time-dependent manner. When p27-pcDNA3.1-K562 cells were treated with STI571, the cell survival rate was markedly decreased compared to K562 cells treated with STI571 or p27-pcDNA3.1-K562 cells, suggesting that p27 and STI571 had a synergistic action on inhibiting K562 cell proliferation (Figure 7).
p27 gene is a member of CDK-interacting protein/kinase inhibitor protein family, and could combine with cyclin-E/CDK2 or cyclin-D/CDK4 to inhibit its kinase activity[18,19]. We constituted a p27-pcDNA3.1 vector and transfected it into K562 cell line and found that the growth rate of p27-pcDNA3.1-K562 cells was apparently lower than that of control K562 cells. Cell cycle analysis indicated that transfected cells were arrested in G0/G1 phase, Western blot analysis showed that cyclin-E protein expression was decreased, suggesting that p27 gene mainly inhibits cyclin-E/CDK2 activity leading to the arrest of K562 cells in G0/G1 phase. Cells could not get across the G1/S checkpoint[20] to enter S phase. As replication of DNA is in S phase, so the decrease of DNA replication inhibited cell growth. p27 and STI571 showed a synergistic action on inhibition of cell proliferation, and the mechanism might be associated with the cell cycle regulating function of STI571. Deininger[21] and Jonuleit[22] discovered that STI571 could down-regulate cyclin-E and cyclin-D and make over-expression of S phase cells return to normal level.
Our experiments demonstrated that p27 combined with STI571 had a synergistic action on induction of apoptosis of K562 cells. There are different opinions on the apoptosis induction by p27. Eymin[23] found that p27 could inhibit apoptosis induced by drugs because leukemia cells could induce hydrolysis of p27 and the hydrolytic products, p23 and p15 had an inhibition function on apoptosis. Barata[24] found that over-expression of p27 could inhibit Bcl-2 expression induced by IL-7, thereby inducing apoptosis of leukemia cells. Patel[25] found that adenovirus-mediated p27 transfection could induce apoptosis of many cancer cell lines, and the results of Schreiber[26] and Craig[27] were similar. So whether p27 promotes or inhibits apoptosis depends on its hydrolytic state, cell types and states. The maladjusted relation of BCR-ABL and p27 plays an important role in the progress of K562 cells. Parada[28] found that BCR-ABL could downregulate p27 protein expression and mRNA level, mainly by inhibiting the phosphatidylinostiol-3-kinase (PI3K) signal pathway and by decreasing the degradation of p27. STI571 could inhibit the expression of some cyclins such as cyclin-D, cyclin-A, cyclin-E, which could relieve the inhibition function of cyclins to p27[29]. So p27 gene transfected into K562 cells not only could reconstruct the regulation of cell cycle pathways, but also strengthen the apoptosis-inducing function of p27 after combining with STI571, suggesting that p27 and STI571 has a synergistic action on apoptosis induction.
p27 gene clone combined with STI571 can not only inhibit cell proliferation and induce apoptosis, but also induce cell differentiation as shown by Fang[30] and Caslini[31] independently. STI571 and p27 gene used in combination may have an important therapeutic significance.
| 1. | Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, Lydon NB. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100-104. [PubMed] |
| 2. | Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2581] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 3. | Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, Gilliland DG, Druker BJ. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood. 1997;90:4947-4952. [PubMed] |
| 4. | McGary EC, Onn A, Mills L, Heimberger A, Eton O, Thomas GW, Shtivelband M, Bar-Eli M. Imatinib mesylate inhibits platelet-derived growth factor receptor phosphorylation of melanoma cells but does not affect tumorigenicity in vivo. J Invest Dermatol. 2004;122:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 932] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 6. | le Coutre P, Tassi E, Varella-Garcia M, Barni R, Mologni L, Cabrita G, Marchesi E, Supino R, Gambacorti-Passerini C. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758-1766. [PubMed] |
| 7. | Hofmann WK, Komor M, Hoelzer D, Ottmann OG. Mechanisms of resistance to STI571 (Imatinib) in Philadelphia-chromosome positive acute lymphoblastic leukemia. Leuk Lymphoma. 2004;45:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2053] [Cited by in RCA: 1942] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 9. | Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 586] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 10. | Koh TY, Park SW, Park KH, Lee SG, Seol JG, Lee DW, Lee CT, Heo DS, Kim KH, Sung MW. Inhibitory effect of p27KIP1 gene transfer on head and neck squamous cell carcinoma cell lines. Head Neck. 2003;25:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Piva R, Cancelli I, Cavalla P, Bortolotto S, Dominguez J, Draetta GF, Schiffer D. Proteasome-dependent degradation of p27/kip1 in gliomas. J Neuropathol Exp Neurol. 1999;58:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Hoos A, Nissan A, Stojadinovic A, Shia J, Hedvat CV, Leung DH, Paty PB, Klimstra D, Cordon-Cardo C, Wong WD. Tissue microarray molecular profiling of early, node-negative adenocarcinoma of the rectum: a comprehensive analysis. Clin Cancer Res. 2002;8:3841-3849. [PubMed] |
| 13. | Kawano T, Horiguchi-Yamada J, Saito S, Iwase S, Furukawa Y, Kano Y, Yamada H. Ectopic cyclin D1 expression blocks STI571-induced erythroid differentiation of K562 cells. Leuk Res. 2004;28:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Hehlmann R, Hochhaus A, Berger U, Reiter A. Current trends in the management of chronic myelogenous leukemia. Ann Hematol. 2000;79:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Amarante-Mendes GP, Jascur T, Nishioka WK, Mustelin T, Green DR. Bcr - Abl-mediated resistance to apoptosis is independent of PI 3-kinase activity. Cell Death Differ. 1997;4:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood. 1998;91:1700-1705. [PubMed] |
| 17. | Li J, Kleeff J, Guo J, Fischer L, Giese N, Büchler MW, Friess H. Effects of STI571 (gleevec) on pancreatic cancer cell growth. Mol Cancer. 2003;2:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 1610] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 19. | Russo AA, Jeffrey PD, Patten AK, Massagué J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 682] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | Pommier Y, Kohn KW. Cell cycle and checkpoints in oncology: new therapeutic targets. Med Sci (Paris). 2003;19:173-186. [PubMed] |
| 21. | Deininger MW, Vieira S, Mendiola R, Schultheis B, Goldman JM, Melo JV. BCR-ABL tyrosine kinase activity regulates the expression of multiple genes implicated in the pathogenesis of chronic myeloid leukemia. Cancer Res. 2000;60:2049-2055. [PubMed] |
| 22. | Jonuleit T, Peschel C, Schwab R, van der Kuip H, Buchdunger E, Fischer T, Huber C, Aulitzky WE. Bcr-Abl kinase promotes cell cycle entry of primary myeloid CML cells in the absence of growth factors. Br J Haematol. 1998;100:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Eymin B, Sordet O, Droin N, Munsch B, Haugg M, Van de Craen M, Vandenabeele P, Solary E. Caspase-induced proteolysis of the cyclin-dependent kinase inhibitor p27Kip1 mediates its anti-apoptotic activity. Oncogene. 1999;18:4839-4847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Barata JT, Cardoso AA, Nadler LM, Boussiotis VA. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1). Blood. 2001;98:1524-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Patel SD, Tran AC, Ge Y, Moskalenko M, Tsui L, Banik G, Tom W, Scott M, Chen L, Van Roey M. The p53-independent tumoricidal activity of an adenoviral vector encoding a p27-p16 fusion tumor suppressor gene. Mol Ther. 2000;2:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Schreiber M, Muller WJ, Singh G, Graham FL. Comparison of the effectiveness of adenovirus vectors expressing cyclin kinase inhibitors p16INK4A, p18INK4C, p19INK4D, p21(WAF1/CIP1) and p27KIP1 in inducing cell cycle arrest, apoptosis and inhibition of tumorigenicity. Oncogene. 1999;18:1663-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee SJ, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997;14:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Parada Y, Banerji L, Glassford J, Lea NC, Collado M, Rivas C, Lewis JL, Gordon MY, Thomas NS, Lam EW. BCR-ABL and interleukin 3 promote haematopoietic cell proliferation and survival through modulation of cyclin D2 and p27Kip1 expression. J Biol Chem. 2001;276:23572-23580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Jonuleit T, van der Kuip H, Miething C, Michels H, Hallek M, Duyster J, Aulitzky WE. Bcr-Abl kinase down-regulates cyclin-dependent kinase inhibitor p27 in human and murine cell lines. Blood. 2000;96:1933-1939. [PubMed] |
| 30. | Fang G, Kim CN, Perkins CL, Ramadevi N, Winton E, Wittmann S, Bhalla KN. CGP57148B (STI-571) induces differentiation and apoptosis and sensitizes Bcr-Abl-positive human leukemia cells to apoptosis due to antileukemic drugs. Blood. 2000;96:2246-2253. [PubMed] |
| 31. | Caslini C, Shilatifard A, Yang L, Hess JL. The amino terminus of the mixed lineage leukemia protein (MLL) promotes cell cycle arrest and monocytic differentiation. Proc Natl Acad Sci USA. 2000;97:2797-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |