Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.1965
Revised: September 22, 2004
Accepted: November 23, 2004
Published online: April 7, 2005
AIM: To investigate the relationship between pancreatic amylase in bile duct and the clinico-pathological features in adult patients with choledochal cyst and anomalous pancreatico-biliary ductal union (APBDU).
METHODS: From 39 patients who underwent surgery for choledochal cyst between March 1995 and March 2003, we selected 15 adult patients who had some symptoms and were radiologically diagnosed as APBDU, and their clinico-pathological features were subsequently evaluated retrospectively. However, we could not obtain biliary amylase in all the patients because of the surgeon’s slip. Therefore, we measured the amylase level in gall bladder of 10 patients and in common bile duct of 11 patients.
RESULTS: Levels of amylase in common bile duct and gall bladder ranged from 11500 to 212000 IU/L, and the younger the patients, the higher the biliary amylase level (r = -0.982, P<0.01). Pathologically, significant correlation was found between the size of choledochal cyst and the grade of inflammation (r = 0.798, P<0.01). And, significant correlation was found between the level of amylase in gall bladder and the grade of hyperplasia. On the other hand, there was no correlation to the age of symptomatic onset or inflammatory grade (r = 0.743, P<0.05). Level of lipase was elevated from 6000 to 159000 IU/L in bile duct and from 14400 to 117000 IU/L in the gall bladder; however, there was no significant correlation with age or clinico-pathological features.
CONCLUSION: The results support the notion that amylase has a particular role in the onset of symptoms, and suggest that a large amount of biliary amylase induces early onset of symptom, thereby making early diagnosis possible.
- Citation: Jeong IH, Jung YS, Kim H, Kim BW, Kim JW, Hong J, Wang HJ, Kim MW, Yoo BM, Kim JH, Han JH, Kim WH. Amylase level in extrahepatic bile duct in adult patients with choledochal cyst plus anomalous pancreatico-biliary ductal union. World J Gastroenterol 2005; 11(13): 1965-1970
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/1965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.1965
Although its etiology has not fully been identified, many theories have been proposed regarding the development of choledochal cyst, and the most popular theory is that a choledochal cyst results from an anomalous pancreaticobiliary ductal union (APBDU)[1-4].
Bile duct dilatation is thought to reflux destructive pancreatic enzymes into the common bile duct via common channel; however, it is not clear which enzymes are mainly causative[5]. Also, it is of a great interest to note that, in the patients with APBDU, the age of symptomatic onsets of various biliary diseases varies. A question of whether pancreatic enzymes in bile duct bring on pathological change of gall bladder or common bile duct has not yet been answered.
Clinical manifestations of choledochal cyst with APBDU in different age groups have been widely studied[6-8], and some reported that inflammatory change of biliary tree is prominent in older patients, and that the level of biliary amylase is higher in adult group than pediatric group. However, low amylase level in biliary tract is natural in neonates or pediatric patients, because of immature exocrine pancreatic function. Therefore, simple comparison between adult and pediatric patients may be misleading. Consequently, we focused on the level of biliary amylase in adult choledochal cyst with APBDU and studied its relationship between the age of symptomatic onset and clinical and pathological features, such as inflammatory change or hyperplasia.
Thirty-nine patients, presenting choledochal cyst, were treated surgically between March 1995 and March 2003. From the 39 patients, we selected 15 patients whose case was radiologically diagnosed as choledochal cyst with APBDU, who were adults, older than 20 years of age and had presented many symptoms. Three were male and 12 were female whose age ranged between 20 and 59 years (mean age was 37.0 years). We excluded 24 patients from these studies who were pediatric patients younger than 20 years or were not radiologically evaluated for APBDU, because we limited the patients to adults with choledochal cyst plus APBDU. Furthermore, we could not obtain biliary amylase in all the patients because of surgeon’s slip. Therefore, we measured the amylase level in gall bladder of 11 patients and in common bile duct of 12 patients.
We reviewed medical records of each patient and analyzed clinical symptoms, age of symptomatic onset, size of choledochal cyst, type of APBDU and level of blood amylase. We also measured the level of amylase and lipase in bile juice taken from gall bladder and common bile duct. These samples were collected at the beginning of the dissection of the cyst before any surgical manipulation.
The pathological findings of these patients were reviewed by a pathologist, except for two patients, because one patient underwent only cholecystectomy and in the other case the gall bladder specimen was not stored. We classified the grade of inflammation from normal to III, which are histopathologically considered as infiltration of inflammatory cells and surface erosion. We also classified the grade of hyperplasia from normal to III, which are histopathologically considered as fractional area of papillary epithelial proliferation from the total epithelium. For histopathologic findings in bile duct, however, accurate evaluation was impossible, because of autolytic change in most of the cases. Because of this reason, histopathologic findings of bile duct were excluded from this study.
Choledochal cyst was classified according to Todani’s classification[9], and APBDU was classified into two types according to Kimura classification[10]; pancreatico-choledochal type (P-C type) and choledocho-pancreatic type (C-P type). Choledochal cyst and APBDU were diagnosed by endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP) and were confirmed by two endoscopic specialists.
For statistical evaluation of the results, Spearman rank correlation coefficient (r) was performed to evaluate the relationship between the age and level of biliary amylase/lipase and between the grade of hyperplasia and level of biliary amylase/lipase, and t-test was performed to evaluate the difference of the grade of inflammation according to grouping the size of cyst by 4 cm. P value <0.05 was considered statistically significant.
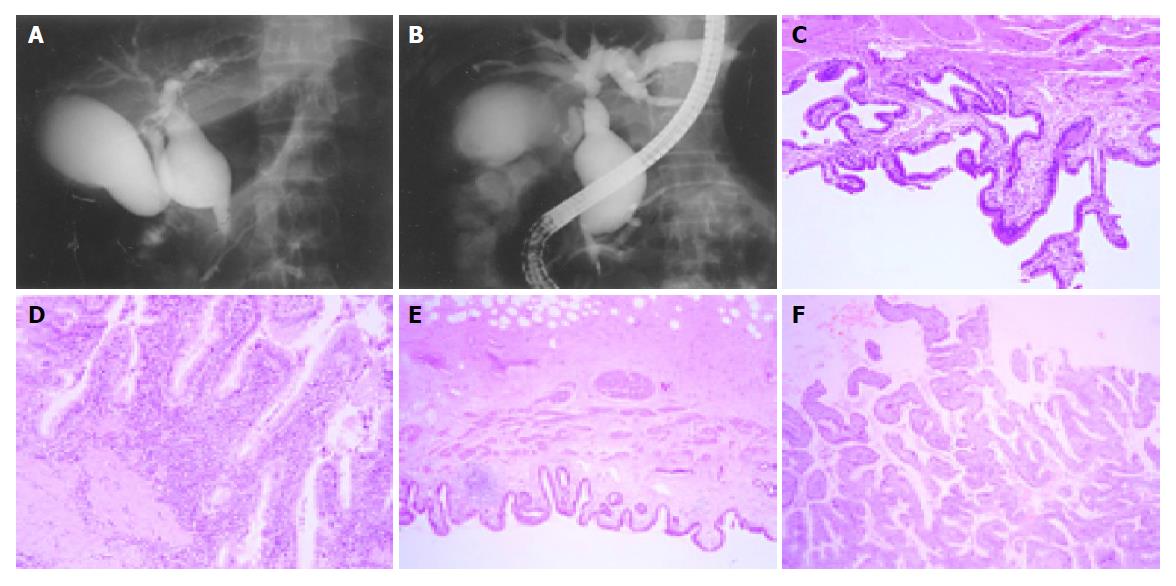
As listed in Table 1 there were 12 female and 3 male patients, and age ranged from 20 to 59 years (mean age 37.0 years). Twelve patients were presented with abdominal pain, three patients presented jaundice with abdominal pain, and none of the patient had indistinct clinical symptom or only jaundice. Eight patients presented type I choledochal cyst (Figure 1A), and seven had type IVa (Figure 1B). The size of choledochal cyst ranged from 2.0 to 10 cm, and mean size was 5.16±2.15 cm. Fourteen patients presented C-P type APBDU, and one presented P-C type APBDU. Gallstone disease was associated in two patients and common bile duct stones were found in four patients. Gall bladder cancer was observed in one patient whose age was 45 years and common bile duct carcinoma was observed in one patient whose age was 59 years. Levels of blood amylase and lipase in all of the patients were in normal range.
| Parameter | n (%) |
| Age (yr) | 15 |
| 2029 yr | 3 (20) |
| 3039 yr | 6 (40) |
| 4049 yr | 4 (27) |
| >50 yr | 2 (13) |
| Sex | 15 |
| Male | 3 (20) |
| Female | 12 (80) |
| Symptom at onset | 15 |
| Indistinct | 0 (0) |
| Jaundice | 0 (0) |
| Abdominal pain | 12 (80) |
| Abdominal pain with jaundice | 3 (20) |
| Size of cyst | 15 |
| ≤4 cm | 8 (53) |
| >4 cm | 7 (47) |
| Type of choledochal cyst | 15 |
| Type I | 8 (53) |
| Type IV | 7 (47) |
| Type of APBDU | 15 |
| Choledocho-pancreatic type | 14 (93) |
| Pancreatico-chole dochal type | 1 (7) |
| Combined biliary disease | 8 |
| Gall bladder stone | 2 (13) |
| Bile duct stone | 4 (27) |
| Gall bladder cancer | 1 (7) |
| Bile duct cancer | 1 (7) |
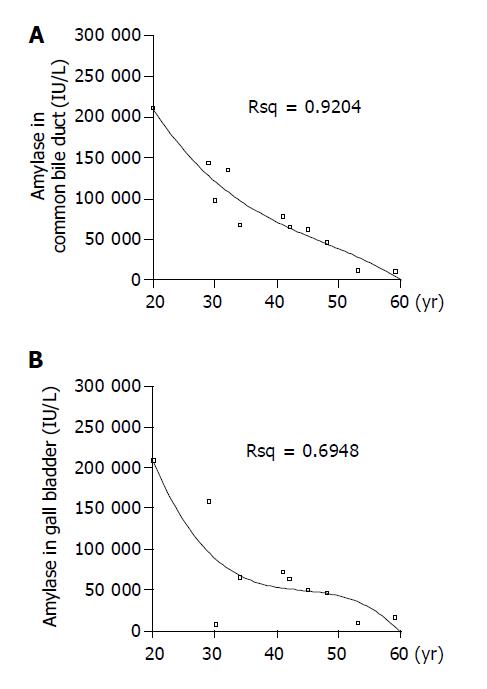
Under general anesthesia, we obtained bile juice from gall bladder and common bile duct, and then measured the levels of amylase and lipase. As listed in Table 2, the level of bile duct amylase was increased, ranging between 11500 and 212000 IU/L, and showed highly significant correlation with the age of symptomatic onset; that is, the younger the patient, the higher the level of amylase in bile duct (Figure 2A) (r = -0.982, P<0.01). And the level of amylase in gall bladder ranged between 11500 and 212000 IU/L, and there was significant correlation with the age of symptomatic onset (Figure 2B) (r = -0.636, P<0.05). The patients with gall bladder carcinoma or bile duct carcinoma had lower level of bile duct amylase (in gall bladder cancer: 62700 IU/L, in bile duct cancer: 11500 IU/L) than the benign cases (mean: 85 345 IU/L). The level of lipase was elevated from 6000 to 159000 IU/L in bile duct and from 14400 to 117000 IU/L in gall bladder; however, there was no significant correlation with age.
| Parameter | Site | n | Range (IU/L) | Mean±SD |
| Amylase | ||||
| Gall bladder | 10 | 11500–212000 | 73148±65244 | |
| Bile duct | 11 | 11500–212000 | 85345±59660 | |
| Lipase | ||||
| Gall bladder | 8 | 14400–117000 | 52175±33791 | |
| Bile duct | 8 | 6000–159000 | 49375±51058 |
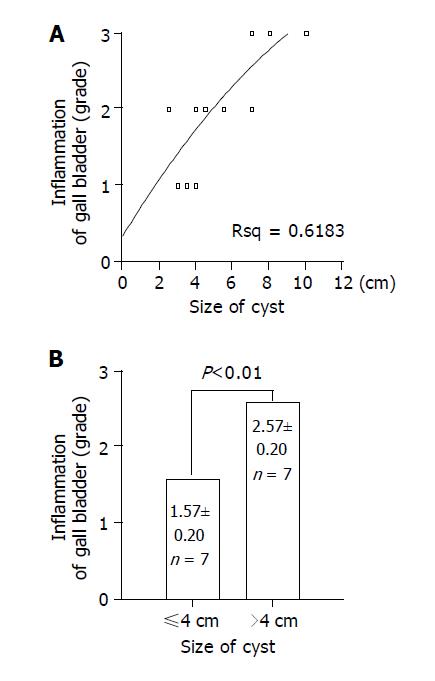
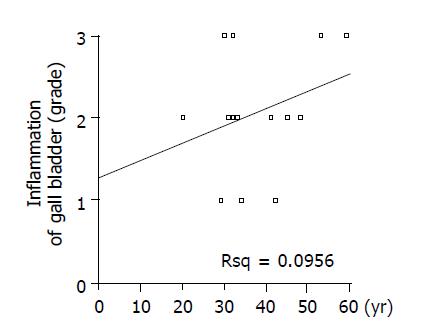
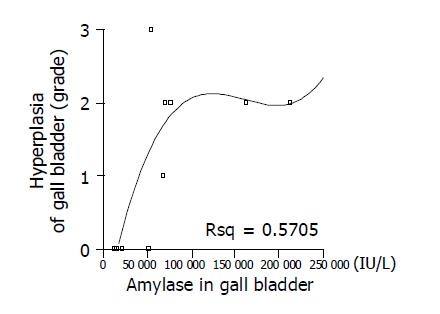
As listed in Table 3, we classified the grade of inflammation of 15 patients from normal to III, which is considered as infiltration of inflammatory cells and surface erosion in histopathologic findings; infiltration of small number of lymphocyte to grade I (Figure 1C), infiltration of moderate number of lymphocytes with multi-focal aggregates to grade II, and extensive infiltration of lymphocytes with erosion of epithelium to grade III (Figure 1D). And we classified the grade of hyperplasia from normal to III, which is considered as fractional area of papillary epithelial proliferation from the total epithelium; less than one-third to grade I (Figure 1E), more than one-third but less than two-thirds to grade II, and more than two-thirds to grade III (Figure 1F). There was no significant correlation among inflammatory change, type of cyst, and biliary amylase (data not shown). Significant correlation was found between the size of choledochal cyst and inflammation (Figure 3A) (r = 0.798, P<0.01), but there was no correlation between age and inflammatory grade of gall bladder (Figure 4) (P>0.05). As the size of choledochal cyst increased, inflammatory grade was raised, and this change was more apparent by grouping the size. In the group of choledochal cyst size less than 4 cm, there was low-grade inflammation, and more severe inflammation in the group with cyst bigger than 4 cm (Figure 3B) (P<0.01). Finally, the grade of hyperplasia in gall bladder was increased with increasing the level of amylase in gall bladder (Figure 5) (r = 0.743, P<0.05).
| Parameter | Site | Grade | n (%) |
| Hyperplasia | |||
| Gall bladder | 14 | ||
| 0 | 5 (36) | ||
| 1 | 2 (14) | ||
| 2 | 6 (43) | ||
| 3 | 1 (7) | ||
| Bile duct | 15 | ||
| 0 | 9 (60) | ||
| 1 | 5 (33) | ||
| 2 | 0 (0) | ||
| 3 | 1 (7) | ||
| Inflammation | |||
| Gall bladder | 14 | ||
| 0 | 0 (0) | ||
| 1 | 3 (21) | ||
| 2 | 7(50) | ||
| 3 | 4 (29) | ||
| Bile duct | 15 | ||
| 0 | 2 (13) | ||
| 1 | 5 (33) | ||
| 2 | 7 (47) | ||
| 3 | 1 (7) |
It has been shown that high biliary amylase level is commonly observed in choledochal cyst with APBDU[11-14]. Oguchi et al[12], studied 40 adults and children with choledochal cyst, and showed correlation of the type of biliary epithelium with the level of amylase in bile, and higher amylase level in a group with abdominal pain than in a group with jaundice. Schweizer and Schweizer[13] and Imazu et al[14], reported that patients with high levels of biliary amylase had a higher pressure difference between the sphincter of Oddi and the duodenum than those with low levels of biliary amylase, and that, in the high amylase group, a spastic sphincter of Oddi plus lack of sphincter function might result in free reflux of pancreatic juice into the biliary system. According to these reports, an elevated level of biliary amylase seems to be a useful biochemical marker of pancreatic reflux into the biliary tree.
Our present results showed significantly high biliary amylase levels in adult patients who had symptomatic onset at young age. Indeed, the biliary amylase level in APBDU patients was inversely proportional to the age of symptomatic onset. This finding is of a great interest and, to the best of our knowledge, this is the first ever documented. The results also support the notion that amylase has a particular role in the onset of symptoms, and suggest that a large amount of biliary amylase induces early onset of symptoms, thereby making early diagnosis possible. On the other hand, a small amount of biliary amylase might result in chronic and insidious clinical course, thus delaying diagnosis and evolving into cancerous change at older age.
However, there are several unanswered questions. First, amylase itself may not be a causative factor of choledochal cyst, and it is not known which pancreatic exocrine enzymes or bile components cause pathological change of bile duct, when the pancreatic juice flows backward. Nevertheless, secondary bile acids, phospholipase A2, trypsin, elastase, and amylase have been proposed by some authors as causative factors for clinical features of choledochal cyst[15-18]. Second, an elevated level of biliary amylase has been considered to be a useful biochemical marker of pancreatic reflux into the biliary tree; however, it is doubtful whether biliary amylase concentration truly reflects the amount of pancreatic free reflux. Therefore, further studies on other pancreatic enzymes, such as trypsin, elastase, chymotrypsin, and phospholipase A2, are necessary. Third, because all of the selected patients presented abdominal pain, we could not study patients with other symptoms such as jaundice.
The level of lipase in bile duct was also elevated. Unlike biliary amylase, there was no significant correlation with age. Nevertheless, Layer et al[19], and Holtmann et al[20], earlier reported that loss of lipase activity correlated with the presence of chymotrypsin, whose activity was associated with the presence of carbohydrate and bile acids, and that lipolytic activity was highly susceptible to inactivation by chymotrypsin or bile acids, but amylase activity was the most stable. In this regard, further studies on other pancreatic enzymes seem to be necessary.
We excluded pediatric patients younger than 20 years from these studies, because we planned the age limit only to adults. In children, there are different levels of biliary amylase because of their immature exocrine pancreatic function. Zoppi et al[21], showed that there are quantitative differences between children and term neonates. Thus, there is a 10-fold difference in duodenal fluid volume, a 7-fold difference in trypsin secretion, a 30-fold difference in lipase secretion, and a 500-fold difference in amylase secretion between children and neonates. The mean pancreatic amylase activity in newborns is 3% of that of adults, begins to increase at 7-8 mo, and reaches adult values by 5 years[22]. Therefore, their biliary amylase level is lower than that in adult patients with choledochal cyst. We also observed such a tendency in neonates (data not shown). Therefore, biliary amylase in very young children appears not to be a reliable biochemical marker of pancreatic reflux, and infants with antenatally diagnosed choledochal cyst have been shown to have low biliary amylase level. It is highly likely that there exists other congenital factors, such as weakness of bile duct or distal obstruction of common bile duct[23].
Amylase levels in gall bladder and common bile duct showed no difference between types I and IV choledochal cysts (data not shown). This is probably due to dilatation of cystic duct, accompanied with formation of choledochal cyst and functional loss of spiral valve, making gall bladder and bile duct to be connected to common chamber and finally the concentration capacity of gall bladder disappears. In one patient with type III choledochal cyst, who had normal sized cystic duct far from choledochal cyst, the level of amylase was more than 8-fold greater in gall bladder than in common bile duct (data not shown).
Pathological findings revealed that the grade of inflammation ranged from normal to severe change, and that the grade of inflammation was related with the size of choledochal cyst, but not with the age of symptomatic onset or with the level of biliary amylase. It seems, therefore, likely that the level of biliary amylase is not intimately related with initiation and progression of inflammatory process, and there is a tendency that the more severe the grade of inflammation, the bigger the size of cyst.
In the present study, the grade of hyperplasia was found to be related with the level of biliary amylase, however, it was not with the age of symptomatic onset and the size of choledochal cyst. Therefore, the hyperplasia in gall bladder seems to be related directly with pancreatic juice reflux, but not with symptomatic onset. The mean age of patients with gall bladder carcinoma has been shown to be significantly lower than that with primary gall bladder carcinoma; about 40-50 years in the case of patients with APBDU vs more than 65 years in the latter[24,25]. It is unlikely that higher biliary amylase levels are related with higher grade of hyperplasia in adult patients who had symptomatic onset as children. Rather, high biliary amylase level may have a particular role in earlier onset of gall bladder carcinogenesis than usual onset of primary gall bladder cancer. Further studies on other pancreatic enzymes as well as relationship between mucosal hyperplasia and carcinoma seem to be necessary.
In conclusion, biliary concentration of pancreatic amylase is closely related with the age of symptomatic onset in adult choledochal cyst with APBDU, and the level of biliary amylase tends to decrease with age, showing inverse proportionality. Therefore, the high level of biliary amylase is suggested as a useful biochemical marker of symptomatic onset. And, hyperplasia of gall bladder was increased with increasing the level of amylase in bile duct, supporting the hypothesis that free reflux plays a particular role in hyperplastic change. Reflux of pancreatic fluid plays not only a particular role in symptomatic onset, but also plays some roles in earlier onset of gall bladder carcinogenesis than usual onset of primary gall bladder cancer. We analyzed only amylase and lipase. However, similar changes might be expected with other pancreatic enzymes such as trypsin, phospholipase and elastase. For better understanding of pathophysiology, further detailed studies should be carried out with a large number of patients.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Kimura K, Ohto M, Saisho H, Unozawa T, Tsuchiya Y, Morita M, Ebara M, Matsutani S, Okuda K. Association of gallbladder carcinoma and anomalous pancreaticobiliary ductal union. Gastroenterology. 1985;89:1258-1265. [PubMed] |
| 2. | Arima E, Akita H. Congenital biliary tract dilatation and anomalous junction of the pancreatico-biliary ductal system. J Pediatr Surg. 1979;14:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Komi N, Tamura T, Miyoshi Y, Kunitomo K, Udaka H, Takehara H. Nationwide survey of cases of choledochal cyst. Analysis of coexistent anomalies, complications and surgical treatment in 645 cases. Surg Gastroenterol. 1984;3:69-73. [PubMed] |
| 4. | Misra SP, Gulati P, Thorat VK, Vij JC, Anand BS. Pancreaticobiliary ductal union in biliary diseases. An endoscopic retrograde cholangiopancreatographic study. Gastroenterology. 1989;96:907-912. [PubMed] |
| 5. | Babbitt DP, Starshak RJ, Clemett AR. Choledochal cyst: a concept of etiology. Am J Roentgenol Radium Ther Nucl Med. 1973;119:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 177] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Davenport M, Stringer MD, Howard ER. Biliary amylase and congenital choledochal dilatation. J Pediatr Surg. 1995;30:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Chaudhary A, Dhar P, Sachdev A, Kumar N, Vij JC, Sarin SK, Broor SL, Sharma SS. Choledochal cysts--differences in children and adults. Br J Surg. 1996;83:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Lai HS, Duh YC, Chen WJ, Chen CC, Hung WT, Lee PH, Huang SF. Manifestations and surgical treatment of choledochal cyst in different age group patients. J Formos Med Assoc. 1997;96:242-246. [PubMed] |
| 9. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 835] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Kimura K, Ohto M, Ono T, Tsuchiya Y, Saisho H, Kawamura K, Yogi Y, Karasawa E, Okuda K. Congenital cystic dilatation of the common bile duct: relationship to anomalous pancreaticobiliary ductal union. AJR Am J Roentgenol. 1977;128:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Iwai N, Yanagihara J, Tokiwa K, Shimotake T, Nakamura K. Congenital choledochal dilatation with emphasis on pathophysiology of the biliary tract. Ann Surg. 1992;215:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Oguchi Y, Okada A, Nakamura T, Okumura K, Miyata M, Nakao K, Kawashima Y. Histopathologic studies of congenital dilatation of the bile duct as related to an anomalous junction of the pancreaticobiliary ductal system: clinical and experimental studies. Surgery. 1988;103:168-173. [PubMed] |
| 13. | Schweizer P, Schweizer M. Pancreaticobiliary long common channel syndrome and congenital anomalous dilatation of the choledochal duct--study of 46 patients. Eur J Pediatr Surg. 1993;3:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Imazu M, Iwai N, Tokiwa K, Shimotake T, Kimura O, Ono S. Factors of biliary carcinogenesis in choledochal cysts. Eur J Pediatr Surg. 2001;11:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Yamashiro Y, Sato M, Shimizu T, Oguchi S, Miyano T. How great is the incidence of truly congenital common bile duct dilatation? J Pediatr Surg. 1993;28:622-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Reveille RM, Van Stiegmann G, Everson GT. Increased secondary bile acids in a choledochal cyst. Possible role in biliary metaplasia and carcinoma. Gastroenterology. 1990;99:525-527. [PubMed] |
| 17. | Shimada K, Yanagisawa J, Nakayama F. Increased lysophosphatidylcholine and pancreatic enzyme content in bile of patients with anomalous pancreaticobiliary ductal junction. Hepatology. 1991;13:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Nakamura T, Okada A, Higaki J, Tojo H, Okamoto M. Pancreaticobiliary maljunction-associated pancreatitis: an experimental study on the activation of pancreatic phospholipase A2. World J Surg. 1996;20:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Layer P, Go VL, DiMagno EP. Fate of pancreatic enzymes during small intestinal aboral transit in humans. Am J Physiol. 1986;251:G475-G480. [PubMed] |
| 20. | Holtmann G, Kelly DG, Sternby B, DiMagno EP. Survival of human pancreatic enzymes during small bowel transit: effect of nutrients, bile acids, and enzymes. Am J Physiol. 1997;273:G553-G558. [PubMed] |
| 21. | Zoppi G, Andreotti G, Pajno-Ferrara F, Njai DM, Gaburro D. Exocrine pancreas function in premature and full term neonates. Pediatr Res. 1972;6:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 201] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Gillard BK, Simbala JA, Goodglick L. Reference intervals for amylase isoenzymes in serum and plasma of infants and children. Clin Chem. 1983;29:1119-1123. [PubMed] |
| 23. | Turnberg LA, Jones EA, Sherlock S. Biliary secretion in a patient with cystic dilation of the intrahepatic biliary tree. Gastroenterology. 1968;54:1155-1161. [PubMed] |
| 24. | Sandoh N, Shirai Y, Hatakeyama K. Incidence of anomalous union of the pancreaticobiliary ductal system in biliary cancer. Hepatogastroenterology. 1997;44:1580-1583. [PubMed] |
| 25. | Voyles CR, Smadja C, Shands WC, Blumgart LH. Carcinoma in choledochal cysts. Age-related incidence. Arch Surg. 1983;118:986-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 144] [Article Influence: 3.4] [Reference Citation Analysis (0)] |