Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.1946
Revised: September 16, 2004
Accepted: November 29, 2004
Published online: April 7, 2005
AIM: Helicobacter pylori (H pylori) is associated with increased gastric inflammatory and epithelial expression of macrophage migration inhibitory factor (MIF) and gastric epithelial cell proliferation. This study aimed at determining whether H pylori directly stimulates release of MIF in monocytes, whether the cag pathogenicity island (PAI) is involved for this function, and whether MIF stimulated by H pylori increases gastric epithelial cell proliferation in vitro.
METHODS: A cytotoxic wild-type H pylori strain (TN2)and its three isogenic mutants (TN2△cag, TN2△cagA and TN2△cagE) were co-cultured with cells of a human monocyte cell line, THP-1, for 24 h at different organism/cell ratios. MIF in the supernatants was measured by an ELISA. Cells of a human gastric cancer cell line, MKN45, were then co-cultured with the supernatants, with and without monoclonal anti-MIF antibody for 24 h. The cells were further incubated for 12 h after addition of 3H-thymidine, and the levels of incorporation of 3H-thymidine were measured with a liquid scintillation counter.
RESULTS: The wild-type strain and the isogenic mutants, TN2△cagA and TN2△cagE, increased MIF release at organism/cell ratios of 200/1 and 400/1, but not at the ratios of 50/1 and 100/1. However, the mutant TN2△cag did not increase the release of MIF at any of the four ratios. 3H-thymidine readings for MKN-45 cells were significantly increased with supernatants derived from the wild-type strain and the mutants TN2△cagA and TN2△cagE, but not from the mutant TN2△cag. Moreover, in the presence of monoclonal anti-MIF antibody, the stimulatory effects of the wild-type strain on cell proliferation disappeared.
CONCLUSION: H pylori stimulates MIF release in monocytes, likely through its cag PAI, but not related to cagA or cagE. H pylori-stimulated monocyte culture supernatant increases gastric cell proliferation, which is blocked by anti-MIF antibody, suggesting that MIF plays an important role in H pylori-induced gastric epithelial cell proliferation.
-
Citation: Xia HHX, Lam SK, Chan AO, Lin MCM, Kung HF, Ogura K, Berg DE, Wong BCY. Macrophage migration inhibitory factor stimulated by
Helicobacter pylori increases proliferation of gastric epithelial cells. World J Gastroenterol 2005; 11(13): 1946-1950 - URL: https://www.wjgnet.com/1007-9327/full/v11/i13/1946.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.1946
Migration inhibitory factor (MIF) plays a pivotal role in inflammatory and immune diseases such as septic shock, rheumatoid arthritis, atopic dermatitis, glomerulonephritis, ulcerative colitis and alcoholic hepatitis[1-4]. In our previous study, MIF was markedly up-regulated during acute gastric ulceration in rats, which was associated with accumulation of macrophages in gastric mucosa[5]. Blockade with the neutralizing anti-MIF antibody inhibited macrophage accumulation and MIF up-regulation, indicating an important pathogenic role of MIF in gastric inflammation and ulceration[5]. Recently, studies have indicated that MIF may also play a critical role as a cytokine in the development of cancers as a link between inflammation and tumorigenesis[1,6,7]. It has been reported that MIF is increasingly expressed in cells of several tumors, including melanoma, neuroblastoma, myelomonocytic leukemia, prostatic cancers of breast, colon, lung, liver, stomach and esophagus, suggesting its involvement in carcinogenesis[1,6-11], although the precise role of MIF in tumor cells remains unclear. In addition, our studies also demonstrated increased serum levels and gastric epithelial expression of MIF in gastric cancer[12].
Helicobacter pylori (H pylori) is a bacterium that colonizes the human stomach and causes gastric inflammation and peptic ulcer disease. Moreover, epidemiological and experimental evidence have proven that infection with H pylori, especially those with cagA gene (a marker of the cag pathogenicity island (PAI)), plays an important role in gastric carcinogenesis[13-16]. We observed that H pylori infection increased MIF expression in both gastric inflammatory and epithelial cells[17]; thus, we hypothesize that H pylori infection may contribute to gastric carcinogenesis, in part, through an MIF-mediated pathway. Therefore, the aim of this study was to determine whether H pylori directly stimulates release of MIF in monocytes, whether the cag PAI is involved in this function, and whether MIF stimulated by H pylori increases gastric epithelial cell proliferation in vitro.
H pylori strain (TN2) and its three isogenic mutants (TN2△cag, TN2△cagA and TN2△cagE) were used in the study. TN2, a strain positive for vacuolating cytotoxin and cag PAI, shares an ancestral strain with TN2GF4, which was previously reported to induce gastric cancer in Mongolian gerbils[16]. THP-1 (American type culture collection, ATCC, Manassas, VA, USA), a human monocyte cell line derived from acute monocyte leukemia and widely applied for in vitro experiments, was used for the determination of the effect of H pylori on MIF release. MKN45 (The RIKEN Cell Bank, The Institute of Physical and Chemical Research, Japan), a poorly differentiated human gastric cancer cell line with wild type p53, was used for the determination of the effect of H pylori-stimulated MIF on gastric cell proliferation.
The bacterial cells of the strain TN2 and its isogenic mutants were cultured on blood agar plates in microaerophilic conditions, and then subcultured in liquid medium for three days, as previously described[18]. The liquid culture was transferred into a 50-mL centrifuge tube, centrifuged at 10000 g for 10 min at 4 °C and washed thrice with 10 mL RPMI 1640. The pellet was suspended in 1 mL RPMI 1640, and frozen at -20°C and thawed thrice at 37 °C. The cells were then sonicated on ice at the maximum power for 12 cycles, with 20 s on and 10 s off in each cycle. The suspension was centrifuged at 12000 g for 15 min at 4 °C, and the supernatant was transferred into an Eppendorf tube, and the whole cell protein content measured using the BCA Protein Assay Kit (Gene Company Limited, Hong Kong, China). The supernatant was kept at -70 °C until use.
THP-1 cells were cultured in RPMI 1640 medium (10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 μg/mL NaHCO3) at 37 °C in a 50 mL/L CO2 - 95 mL/L O2 incubator, collected by using trypsin when they reached 80% confluence, and then adjusted to the concentration of 1×105 cells/mL. The cells (100 μL) were added into wells of 24-well microtiter tray, and then incubated at 37°C for 12 h. The whole cell protein preparations (diluted in RPMI 1640 medium with a final concentration of 40 μg/mL), live H pylori organisms (at organism/cell ratios of 50/1, 100/1, 200/1 and 400/1) were added to THP1 culture. Lipopolysaccharide (Sigma Chemical Co., St. Louis, MO), known to activate THP-1 cells and stimulate MIF release[19,20], was used as a positive control, and RPMI 1640 medium was used as a negative control. In our pilot experiments, the concentration of 1 ng/mL was found to be the lowest concentration that reached the maximum stimulatory effect, and thus was used in the further study. THP1 cells were further incubated for 24 h, and culture supernatants were collected at 6, 12 and 24 h, and stored at -20 °C after centrifugation until use.
MIF in the supernatants was measured by an ELISA according to the manufacturer’s instruction (R&D Systems, Inc., Minneapolis, MN). Briefly, 100 μL of capture anti-human MIF antibody (2 μg/mL) in phosphate-buffered saline (PBS, pH7.4) was transferred into each well of an ELISA plate, blocked with 1% BSA. Hundred microliters of each sample, or recombinant human MIF (R&D Systems, Inc.) were added, in triplicate, into corresponding wells for 2 h at room temperature. Biotinylated anti-human MIF antibody (0.2 μg/mL, R&D Systems, Inc.) was added, and incubated for 2 h at room temperature. After plates were washed thrice, 100 μL streptavidin-HRP (R&D System, Inc.) at a dilution of 1 in 200 was added into each well. Following further washing for four times, 100 μL of 1:1 mixture of reagents A (H2O2) and B (tetramethylbenzidine) was added. Finally, the reaction was stopped by adding 50 μL of H2SO4. Absorbance was read using a microtiter plate reader (NovaPathTM, BIO-RAD, Hercules, CA) set at 450 nm.
After MKN-45 cells were cultured in RPMI 1640 medium at 37 °C for 24 h to reach a confluent monolayer, the above-prepared THP1 culture supernatants were added with a final concentration of 20% in the medium, with or without monoclonal anti-MIF antibody (1 ng/mL, R&D System, Inc.). RPMI 1640 medium was used as a negative control. The cells were further incubated for 24 h, and then 3H-thymidine (50 μc) was added to the medium, and the cells were incubated for additional 12 h. The levels of incorporation of 3H-thymidine of the MKN-45 during the last 12 h were determined in a liquid scintillation counter (Beckman LS 6500, Beckman Instruments, Inc., Fullerton, CA) as counts per minute (cpm).
Data obtained from the study were expressed as the mean±SD. Statistical analysis was performed using SPSS software (version 10.0, SPSS Inc., Chicago, IL). Independent samples t-test, or the Mann-Whitney U test, where appropriate, was used to determine the differences in numeric variables. P values calculated were two-tailed. The alpha level of significance was set at P<0.05.
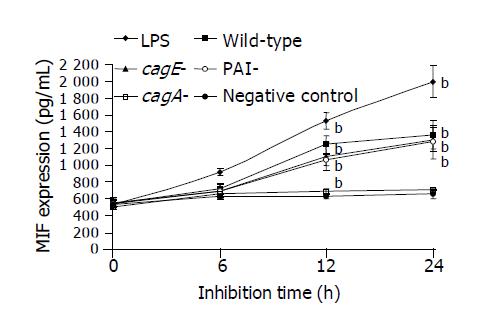
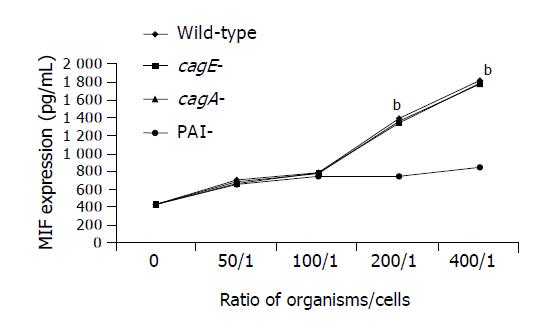
Secretion of MIF by THP-1 cells was significantly increased by the whole cell proteins of the wild-type TN2 strain, cagA-deleted or cagE-deleted mutants in a time-dependent manner (Figure 1). However, the whole cell proteins of PAI-deleted mutants did not have the stimulatory effect on MIF secretion (Figure 1). Similarly, co-culture of live organisms of the wild-type strain, cagA-deleted or cagE-deleted mutants significantly increased MIF secretion in a dose-dependent manner (Figure 2). However, the PAI-deleted mutant had no effect on MIF secretion (Figure 2).
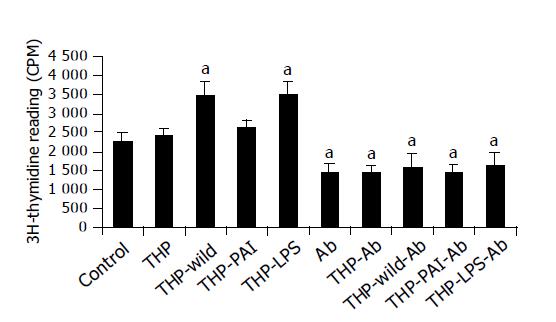
Proliferation of MKN-45 cells was significantly increased by adding the supernatant of co-culture of THP-1 with LPS or with the wild-type strain (Figure 3). The supernatant of co-culture of THP-1 with the PAI-deleted mutant only slightly increased cell proliferation (Figure 3). Interestingly, addition of monoclonal anti-MIF antibody decreased cell proliferation of the gastric cancer cells. Furthermore, the stimulatory effect of LPS and the wild-type H pylori strain on the cell proliferation was completely abolished by the addition of the antibody (Figure 3).
The present study shows that either the whole cell proteins or live organisms of the cytotoxic H pylori strain stimulate the monocytes to release MIF. In addition, the cag PAI-deleted mutant has little effect on the MIF release, suggesting the essential role of PAI in the stimulation of MIF release. Moreover, the supernatant of H pylori co-cultured monocytes increases proliferation of gastric cancer cells and neutralizing monoclonal anti-MIF antibody reduces cell proliferation, indicating that MIF plays an important role in gastric cell proliferation.
Studies have shown that MIF is a pro-inflammatory cytokine and is constitutively expressed, stored in a preformed manner in cytoplasm, and released into circulation upon stimulation[21]. It has been reported that normal gastric epithelial cells constitutively, albeit weakly, express MIF[5,17,22]. In our previous study, we observed that MIF expression was significantly increased in gastric epithelial cells in the presence of H pylori infection[17]. More importantly, it is well known that preformed MIF protein is present in both resting macrophages and monocytes, indicating that the macrophage is not only a target, but also a source of MIF in vivo[2,21,23,24]. It is well established that H pylori infection is associated with gastric infiltration of acute and chronic inflammatory cells, including microphages and monocytes. Previously, we applied a double staining technique to simultaneously detect activated T cells (CD45RO) and macrophages (KP-1) and observed increased MIF expression in these cells in gastric mucosa[17]. In present in vitro study, we clearly show that H pylori stimulated release of MIF by monocytes, most likely through its cag PAI. However, both cagA and cagE appeared not to play a role in the stimulatory effect, since cagA- or cagE-deleted mutant possess the similar stimulatory effect on MIF release to their parent. MIF may play an important role in gastric inflammation and injury. We hypothesize that colonization of H pylori in the stomach directly stimulates the gastric epithelial cells to release MIF, which then promotes local infiltration of inflammatory cells including neutrophils, T- and B- lymphocytes and macrophages, resulting in the production of inducible nitrite oxide synthase (iNOS) and cytotoxic cytokines such as TNF-α, IFN-γ and ICAM-1[1,5,19,25,26]. Consequently, activated T-cells and macrophages per se release a large amount of MIF. In the presence of chronic H pylori infection, this cycle continues, which contributes to more severe inflammation, and gastric injury such as gastric erosion and ulcerations.
Recently, we demonstrated that MIF expression was significantly increased in precancerous lesions and gastric cancer, suggesting its role in gastric carcinogenesis[12]. MIF could be involved in carcinogenesis, by promoting cell proliferation, tumor angiogenesis and metastasis [1,8,26-30]. In previous animal studies, it was observed that increased MIF expression was associated with enhanced proliferation of murine colon cancer cells in response to growth factors and neutralizing anti-MIF-antibodies dramatically reduce the initial outgrowth of 38C13 B cell lymphoma cells in mice[26,27]. MIF has been identified as an angiogenic factor and neutralization of MIF by anti-MIF antibodies inhibits endothelial cell growth and leads to a reduced number of tumor capillaries[27,28]. Inhibition of angiogenesis by anti-MIF antibody treatment has also been reported in a human melanoma model and in murine colon carcinoma cells[8,26,29]. Both increased proliferation of tumor cells and angiogenesis would inevitably result in metastasis. In the present study, we observed that supernatant of monocytes after co-culture with H pylori increased proliferation of gastric cancer cells, and addition of anti-MIF antibody blocked the increase in cell proliferation, suggesting MIF stimulated by H pylori plays an important role in gastric epithelial cell proliferation. How MIF regulates cell proliferation is under intensive investigation. Recent studies suggest that modulation of cell proliferation by MIF could involve a complex regulatory system, in which proteins p53, JAB/CSN5/p27Kip1 and ERK1/2, and possibly other signalosome proteins, may play important roles[1,6,7,30,31].
Genetic analysis reveals that H pylori strains can be divided into two types, by the presence or absence of a 40 kb of foreign DNA, i.e., the cag PAI which consists of at least 18 open-reading frames such as cagA, cagE, etc.[32]. Over the past decade, cagA, a 120-128 ku protein, has been identified as a marker for the presence of cag PAI[33,34]. Upon contact with gastric epithelial cells, cagA is translocated into gastric epithelial cells, where cagA undergoes tyrosine phosphorylation and recruits and activates Src homology phosphatase 2, thereby inducing a growth-factor-like effect[34]. Recent studies have indicated that cagE and several other genes of cag PAI code a type IV secretion system, which is involved in the translocation of cagA protein into the gastric epithelial cells[34-36]. Moreover, isogenic mutant studies demonstrated that some cag PAI genes, including cagE, are essential for interleukin-8 induction and nuclear factor-κB activation[32,34,37,38]. However, a recent study found that the association between cagE genotype and IL-8 induction was weak and implied that gastric cell factors or other bacterial factors are involved in the induction of IL-8[39]. In animal studies, it was observed that knocking out of cagE gene deprived wild-type H pylori of the pathogenicity for gastritis and gastric ulcer in Mongolian gerbils, suggesting that this gene is responsible for various gastric lesions[40,41]. Day et al[42], reported that cagE is associated with H pylori-induced duodenal ulceration in children in Canada. However, studies in Japan and Taiwan showed that presence of cagE appeared not to be related to the severity of gastric mucosal inflammation and ulceration in patients with upper gastroduodenal symptoms since almost all isolates were cagE positive[43-45]. In the present study, we demonstrated that cag PAI is essential for the stimulation of MIF secretion. However, neither cagA nor cagE is involved in the stimulation, suggesting that genes other than cagA and cagE in the cag PAI are responsible for this function.
In conclusion, H pylori stimulates MIF release in monocytes, likely through its cag PAI, but not related to cagA and cagE. H pylori-stimulated monocyte culture supernatant increases gastric cell proliferation, which is blocked by anti-MIF antibody, suggesting that MIF plays an important role in H pylori-induced gastric epithelial cell proliferation.
The authors wish to thank Dr. Xing-Xiang He of the Department of Medicine, The Second Affiliated Hospital of Guangzhou Medical College, Guangzhou, China for performing the experiments. Part of the results in the study was presented at the Digestive Disease Week of the American Gastroenterological Association, Orlando, 17-22 May 2003. Gastroenterology 2003; 124: A269 and Gastroenterology 2003; 124: A594.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Lan HY, Yang N, Nikolic-Paterson DJ, Yu XQ, Mu W, Isbel NM, Metz CN, Bucala R, Atkins RC. Expression of macrophage migration inhibitory factor in human glomerulonephritis. Kidney Int. 2000;57:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M, ten Hove T. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 239] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Kumagi T, Akbar F, Horiike N, Onji M. Increased serum levels of macrophage migration inhibitory factor in alcoholic liver diseases and their expression in liver tissues. Clin Biochem. 2001;34:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Huang XR, Chun Hui CW, Chen YX, Wong BC, Fung PC, Metz C, Cho CH, Hui WM, Bucala R, Lam SK. Macrophage migration inhibitory factor is an important mediator in the pathogenesis of gastric inflammation in rats. Gastroenterology. 2001;121:619-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Mitchell RA, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor (MIF). Semin Cancer Biol. 2000;10:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 493] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 8. | Bin Q, Johnson BD, Schauer DW, Casper JT, Orentas RJ. Production of macrophage migration inhibitory factor by human and murine neuroblastoma. Tumour Biol. 2002;23:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Meyer-Siegler KL, Bellino MA, Tannenbaum M. Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer. 2002;94:1449-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Akbar SM, Abe M, Murakami H, Tanimoto K, Kumagi T, Yamashita Y, Michitaka K, Horiike N, Onji M. Macrophage migration inhibitory factor in hepatocellular carcinoma and liver cirrhosis; relevance to pathogenesis. Cancer Lett. 2001;171:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Xia HH, Zhang ST, Lam SK, Lin MC, Kung HF, Wong BC. Expression of macrophage migration inhibitory factor in esophageal squamous cell carcinoma and effects of bile acids and NSAIDs. Carcinogenesis. 2005;26:11-15. [PubMed] |
| 12. | He XX, Xia HHX, Yang Y, Lam SK, Lin MCM, Wong WM, Kung HF, Leung SY, Yuen ST, Zhao YH. Increased serum levels and epithelial expression of macrophage migration inhibitory factor in gastric cancer. Gastroenterology. 2003;124:A556. [DOI] [Full Text] |
| 13. | Xia HHX, Wong BCY, Lam SK. Helicobacter pylori infection and gastric cancer. Asian J Surg. 2001;24:217-221. |
| 14. | Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 17. | Xia HH, Lam SK, Huang XR, Wong WM, Leung SY, Yuen ST, Lan HY, Wong BC. Helicobacter pylori infection is associated with increased expression of macrophage migratory inhibitory factor--by epithelial cells, T cells, and macrophages--in gastric mucosa. J Infect Dis. 2004;190:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Xia HX, English L, Keane CT, O'Morain CA. Enhanced cultivation of Helicobacter pylori in liquid media. J Clin Pathol. 1993;46:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Pérez-Pérez GI, Shepherd VL, Morrow JD, Blaser MJ. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect Immun. 1995;63:1183-1187. [PubMed] |
| 20. | Nishihira J, Koyama Y, Mizue Y. Identification of macrophage migration inhibitory factor in human leukemia HL-60 cells and its induction by lipopolysaccharide. Biochem Mol Biol Int. 1996;40:861-869. [PubMed] |
| 21. | Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci USA. 1998;95:11383-11388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Maaser C, Eckmann L, Paesold G, Kim HS, Kagnoff MF. Ubiquitous production of macrophage migration inhibitory factor by human gastric and intestinal epithelium. Gastroenterology. 2002;122:667-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 788] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 24. | Lan HY, Bacher M, Yang N, Mu W, Nikolic-Paterson DJ, Metz C, Meinhardt A, Bucala R, Atkins RC. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med. 1997;185:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Swope MD, Lolis E. Macrophage migration inhibitory factor: cytokine, hormone, or enzyme? Rev Physiol Biochem Pharmacol. 1999;139:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Takahashi N, Nishihira J, Sato Y, Kondo M, Ogawa H, Ohshima T, Une Y, Todo S. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol Med. 1998;4:707-714. [PubMed] |
| 27. | Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181-191. [PubMed] |
| 28. | Yang Y, Degranpré P, Kharfi A, Akoum A. Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab. 2000;85:4721-4727. [PubMed] |
| 29. | Ogawa H, Nishihira J, Sato Y, Kondo M, Takahashi N, Oshima T, Todo S. An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine. 2000;12:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, Johannes FJ, Roger T, Calandra T, Kapurniotu A. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 469] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 481] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 32. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1392] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 33. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 933] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 34. | Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 585] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 35. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 449] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 36. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 966] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 37. | Tummuru MK, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 262] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Owen RJ, Sharp S, Lawson AJ, Durrani Z, Rijpkema S, Kidd M. Investigation of the biological relevance of Helicobacter pylori cagE locus diversity, presence of CagA tyrosine phosphorylation motifs and vacuolating cytotoxin genotype on IL-8 induction in gastric epithelial cells. FEMS Immunol Med Microbiol. 2003;36:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Akanuma M, Maeda S, Ogura K, Mitsuno Y, Hirata Y, Ikenoue T, Otsuka M, Watanabe T, Yamaji Y, Yoshida H. The evaluation of putative virulence factors of Helicobacter pylori for gastroduodenal disease by use of a short-term Mongolian gerbil infection model. J Infect Dis. 2002;185:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Day AS, Jones NL, Lynett JT, Jennings HA, Fallone CA, Beech R, Sherman PM. cagE is a virulence factor associated with Helicobacter pylori-induced duodenal ulceration in children. J Infect Dis. 2000;181:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Sheu SM, Sheu BS, Yang HB, Li C, Chu TC, Wu JJ. Presence of iceA1 but not cagA, cagC, cagE, cagF, cagN, cagT, or orf13 genes of Helicobacter pylori is associated with more severe gastric inflammation in Taiwanese. J Formos Med Assoc. 2002;101:18-23. [PubMed] |
| 44. | Sadakane Y, Kusaba K, Nagasawa Z, Tanabe I, Kuroki S, Tadano J. Prevalence and genetic diversity of cagD, cagE, and vacA in Helicobacter pylori strains isolated from Japanese patients. Scand J Gastroenterol. 1999;34:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Kawamura O, Murakami M, Araki O, Yamada T, Tomizawa S, Shimoyama Y, Minashi K, Maeda M, Kusano M, Mori M. Relationship between gastric disease and deletion of cag pathogenicity island genes of Helicobacter pylori in gastric juice. Dig Dis Sci. 2003;48:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |