Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.1922
Revised: October 20, 2004
Accepted: November 29, 2004
Published online: April 7, 2005
AIM: Predictive value of serum b2-microglobulin (b2m) levels for virological breakthrough (VB) in HBeAg-negative chronic hepatitis B (CHB) patients under long-term treatment schedules including lamivudine (LAM).
METHODS: Serum b2m levels were calculated during treatment in 25 CHB patients under long-term LAM monotherapy (group A) and 12 patients under initial interferon plus LAM treatment followed by LAM monotherapy (group B), using the MEIA technology. We used Cox proportional hazard models in order to investigate the association between serum b2m levels and VB.
RESULTS: Seven of 25 patients (28%), 9/25 (36%) and 14/25 (56%) from group A and 0/12, 2/12 (16.6%) and 3/12 (25%) from group B exhibited VB at months 12, 24 and 36 of treatment, respectively. All patients, from both groups, who did not show VB exhibited b2m elevation in mo 3. The duration of b2m elevation was significantly longer in the virological responder’s subgroup from group A than the non-responder’s one (7.3±2.6 vs 3.8±3.4 mo, P = 0.02). In comparison to group A patients whose b2m levels were increased at 3 mo, patients whose b2m levels were decreased had 4.6 times higher risk of experiencing VB (RR = 4.6, P = 0.024). When baseline variables were simultaneously included in the same Cox model, decreased b2m status was still associated with increased risk of VB (RR = 12.2, P = 0.03).
CONCLUSION: In HBeAg-negative CHB patients under either long-term LAM monotherapy or initial combination treatment, serum b2m levels at 3 mo of treatment, compared to baseline ones, might be a predictor of risk for VB.
- Citation: Elefsiniotis IS, Moulakakis A, Pantazis KD, Glynou I, Ketikoglou I, Vezali E, Kada H, Tsianos E. Relationship between serum b2-microglobulin levels and virological breakthrough in HBeAg-negative chronic hepatitis B patients, under long-term treatment schedules including lamivudine. World J Gastroenterol 2005; 11(13): 1922-1928
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/1922.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.1922
Chronic hepatitis B (CHB) is a common disease with an estimated global prevalence of 350 million chronic infected patients according to the World Health Organization (WHO) figure[1]. Hepatitis B e antigen (HBeAg)-negative CHB accounts for 7-30% of patients with CHB worldwide, with the highest rates reported in Mediterranean Europe and Asia[2]. It results from infection with hepatitis B virus (HBV) mutants that are unable to produce HBeAg[3]. In Greece, it is estimated that more than 95% of patients with HBeAg-negative CHB are infected with the precore mutant HBV variant[4]. These patients often appear to have more severe liver disease than that observed in patients infected with the wild type form of the virus and treatment with interferon alpha (IFN-α) is associated with sub-optimal responses, high relapse rates and poor compliance[5,6].
Lamivudine (LAM) is an oral nucleoside analogue that has potent antiviral properties against HBV and human immunodeficiency virus (HIV). Studies in HBeAg-positive[7] and HBeAg-negative[8] CHB patients demonstrate that LAM treatment results in a rapid and consisted suppression of serum HBV-DNA levels with normalization of aminotransferases and significant improvement of liver histology in the majority of patients. Extended LAM treatment increases the emergence of HBV variants, which have changes in the tyrosine-methionine-aspartate-aspartate amino acid (YMDD) locus of the HBV-DNA polymerase sequence, and exhibits reduced susceptibility to the drug[9-12]. Response rates tend to decrease with the prolongation of LAM monotherapy and breakthroughs due to YMDD mutants accumulate, culminating in the development of biochemical breakthroughs in most HBeAg-negative patients[11]. Combination therapy is the treatment of choice for chronic hepatitis C-infected patients by increasing the response rates as well as for HIV-infected ones by reducing the emergence of drug resistance. Recently, Santantonio et al[13], observed that combination treatment (LAM plus interferon) in HBeAg-negative CHB patients was as beneficial as LAM monotherapy, but the combination regimen appeared to delay or prevent the emergence of HBV-DNA polymerase mutants. However, the clinical impact of these mutations is still controversial[9-11] and the prediction of virological breakthrough (VB) is an issue needing further investigation.
B2-microglobulin (b2m) plays a key role in influencing the immune response to viral infections, as it is an integrating part of the human leukocyte antigens (HLA) system[14].In comparison to controls, patients with chronic viral hepatitis manifest enhanced hepatocellular display of class I HLA antigens together with rising serum b2m levels[14,15]. Both hepatic class I HLA antigens and serum b2m levels correlated positively with duration and severity of liver disease[14,15]. Moreover, interferon treatment significantly increases serum b2m levels, offering a Th1-dominant environment to patients with chronic viral hepatitis, irrespective of therapy outcome[15-17]. However, at least in two studies, the hepatocyte b2m expression significantly decreased after interferon treatment[18,19] following the reduction of histological activity of liver disease. The alterations of serum b2m levels in CHB patients under either long-term LAM treatment or interferon plus LAM combination treatment have not been investigated yet.
In this study, we sought to record the alterations of serum b2m levels in HBeAg-negative CHB patients under either long-term LAM monotherapy or interferon plus LAM combination treatment for the first 6 mo followed by LAM monotherapy thereafter and to investigate their association with biochemical and virological outcome data. We hypothesized that serum b2m levels may be a predictor of virological and/or biochemical breakthrough.
Between March 1998 and August 1999, a total of 37 consecutive hepatitis B surface antigen positive (HBsAg+) naive patients (33 males, 4 females) were enrolled prospectively in this pilot study and randomized in the two treatment arms (randomization 2:1). Twenty-five patients were treated with LAM (100 mg daily, per os) for 36 mo(group A) and 12 patients were treated with interferon-alpha 2a (4.5 MU t.i.w, subcutaneously) plus LAM (100 mg daily, per os) for the first 6 mo followed by LAM monotherapy thereafter (group B). All patients had a liver biopsy showing active HBV-related disease within 6 mo before admission to the study. A single pathologist evaluated all biopsy specimens that were scored according to Ishak scoring system (grade = 0-18, stage = 0-6)[20]. Patients were evaluated clinically, biochemically and serologically at entry and every 3 mo during treatment. In order to allow for reasonable enough time for breakthrough to occur, only patients with at least 18 mo of follow-up were included in the current analyses.
To be eligible, patients had to fulfill the following criteria: (1) age greater than 18 years; (2) detectable HBsAg in serum for at least 6 mo; (3) HBeAg negativity; (4) antibody to hepatitis B e antigen (anti-HBe) positivity, with signs of active viral replication (serum HBV-DNA positive); (5) serum alanine aminotransferase (ALT) levels above the normal range at least on two separate occasions in the previous 6 mo and (6) absence of immunomodulatory and/or antiviral treatment for hepatitis in the past. HBV sequencing to confirm genotypic precore mutations was not performed. Patients were excluded if they were infected with hepatitis C (HCV) and/or hepatitis D virus (HDV), human immunodeficiency virus (HIV), had decompensated liver disease (Child-Pugh B or C), had evidence of autoimmune hepatitis (anti-nuclear antibody titer ≥1/160) or had a positive clinical history for other chronic liver disease. Moreover, patients were excluded from the study, if they had a positive clinical history for other chronic diseases or if they were under any other medication. Pregnant and breastfeeding women were also excluded.
Routine biochemical and hematological tests were performed using automated techniques. Virological evaluation (serum HBV-DNA) was done at baseline and at months 6, 12, 24 and 36 of treatment. HBsAg, HBeAg, anti-HBe, anti-HBs (antibody to hepatitis B surface antigen) and HCV, HDV, HIV serological markers were detected using routine commercially available enzyme immunoassays (Abbott Laboratories, Abbott Park, IL, USA). HBV-DNA was quantified with the use of a commercially available polymerase chain reaction (PCR) assay (Amplicor, Roche, Basel, Switzerland) with a lower limit of quantification of 400 copies/mL. Detection of tyrosine-methionine-aspartate-aspartate amino acid HBV-DNA polymerase (YMDD) variants was performed as described by Lai et al[7], using a restriction fragment-linked polymorphism assay, in the proportion of patients who developed VB during the treatment period.
VB was defined as an HBV-DNA reappearance (HBV-DNA>400 copies/mL) during treatment, after at least one previous test had been under detection limit (HBV-DNA≤400 copies/mL).
Biochemical breakthrough (BB) was defined as an ALT flare (ALT>40 IU/L) during treatment, after at least one previous test had been under normal range (ALT≤40 IU/L).
Serum was collected from patients at baseline and every 3 mo during treatment and was stored at -85 °C. Serum b2m levels were calculated using the microparticle enzyme immunoassay (MEIA) technology, which uses submicron microparticles, coated with a capture molecule specific for the analyte being measured (Abbott Laboratories). Serum sample and microparticles (captured molecules) are transferred to the incubation well of the reaction cell. During the incubation period analytes bind to the microparticles, creating an immune complex. The reaction mixture is aspirated from the incubation well to another cell where it is washed in order to remove unbound materials. Then alkaline phosphate (AP)-labeled conjugate is transferred to the cell, which binds to the immune complex to complete the antibody-analyte-conjugate “sandwich”. The new complex is being washed again and then the dispenser adds the substrate 4-methylumbelliferyl phosphate (MUP). The AP conjugate catalyzes the hydrolysis of MUP to 4-methylumbelliferone (MU). The rate at which MU is generated on the cell is proportional to the concentration of analyte in the serum sample.
Written informed consent was obtained from each patient for his or her participation in the study. The study was reviewed and approved by the local ethics committee. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki.
Serum b2m levels were treated as a continuous variable in initial analyses. We subsequently calculated two variables relating to b2m: (1) a continuous variable reflecting the number of 3-mo interval for which b2m was rising, in relation to baseline levels and (2) a dichotomous variable reflecting status of either b2m elevation or decline at 3-mo interval (again in relation to baseline value).
Age, serum ALT levels and histological activity index (HAI grade) of liver disease were all treated as continuous variables in the analyses. Initial serum HBV-DNA levels were dichotomized into two groups: (1) high viral load (>106 copies/mL) and (2) low viral load (≤106 copies/mL).
Student’s t-test and χ2 analyses were used to examine the association between the presence of VB and other potentially interactive variables, including age, ALT levels, BB, grade (HAI), serum HBV-DNA levels and serum b2m levels. A probability value less than 0.05 (P-value <0.05) was considered significant. All data are expressed as mean±SD.
We used survival analyses (Cox proportional hazard Lawless J. Statistical Model and Methods for Lifetime Data New York, NY: John Wiley & Sons Inc. 1982, using the SPSS 12.0 computer software, Lead Technologies Inc.) in order to investigate the association between serum b2m levels (predictor) and VB (outcome). Participants were considered to have failed at the time of the first follow-up visit at which VB was documented. For participants who did not manifest VB, the last follow-up evaluation was used for censoring. Therefore, the Cox model’s time axis was the duration of follow-up until either VB or last evaluation without VB. The initial Cox proportional hazard models used either duration of b2m elevation (continuous variable) or elevation vs decline status at 3-mo interval of b2m (dichotomous variable) as predictor. In subsequent Cox models, we simultaneously included potential confounders (age, ALT, HBV-DNA, HAI) along with b2m status at 3-mo of follow-up in the same Cox model.
All CHB patients tolerated therapy well and completed the treatment period. None of the patients was lost from follow-up. Flu-like syndrome during the first week of treatment, fatigue and mild to moderate mood disorders were observed in the majority of patients from group B and interferon dose reduction was required in four of them (33%) due to neutropenia and/or thrombocytopenia. No relevant side effects were noted in patients from group A. Mild elevation of serum amylase levels, with no clinical significance, was observed in 3 out of 25 (12%) LAM-treated patients. One patient from group A developed a macular skin rash during the first two weeks of treatment, which disappeared automatically in the following two weeks, while he was on LAM treatment. None of the treated CHB patients lost HBsAg and none showed HBsAg seroconversion on the treatment period.
The demographic, laboratory and histological features of both groups of CHB patients at entry are shown in Table 1. As we can see, the two groups were comparable for all the baseline values, including baseline serum b2m levels. Moreover, the mean duration (in months) of serum b2m elevation was comparable between the two groups, whereas the percentage of patients who exhibited serum b2m elevation, at mo 3 of treatment, from group B was significantly higher than the corresponding one from group A (100% vs 68%, P = 0.03). There was no association between histological findings (grade, stage) and either baseline serum ALT levels (P = 0.719 and P = 0.486, respectively) or serum HBV-DNA levels (P = 0.784 and P = 0.542, respectively). Patients with higher level of fibrosis (stage>3) at entry were older than those with fibrosis score≤3 (P = 0.021). Three out of 25 CHB patients (12%) from group A and 2 out of 12 CHB patients (16.6%) from group B had evidence of cirrhosis with ongoing inflammatory activity at baseline liver biopsy (Child-Pugh A).
| Group A (n = 25) | Group B (n = 12) | P | |
| Age (yr) | 42.8±13.6 | 40.8±10.4 | 0.49 |
| ALT (IU/L) | 218±106.1 | 134.6±92.9 | 0.07 |
| HBV-DNA -log10 (copies/mL) | 5.96±0.86 | 6.27±0.76 | 0.29 |
| Grade (0–18) | 7.0±3.3 | 5.6±1.8 | 0.12 |
| Stage (0–6) | 3.2±1.5 | 2.9±1.2 | 0.54 |
| Cirrhosis (%) | 3/25 (12) | 2/12 (16.6) | 0.71 |
| B2m (mg/dL) (baseline levels) | 1695.5±348.1 | 1787.1±367.2 | 0.25 |
| Duration of b2m elevation (mo) | 5.3±3.5 | 5.0±1.47 | 0.82 |
| B2m elevation in 3 mo (%) | 17/25 (68) | 12/12 (100) | 0.03a |
Both serum ALT levels and serum HBV-DNA titers showed a sharp decline during the first 6 mo of therapy in both groups. Serum ALT levels were under the upper normal limits (<40 IU/L) and serum HBV-DNA levels were negative (<400 copies/mL) in all CHB patients who participated in the study, at month 6 of treatment. In the first year of treatment, 7 out of 25 (28%) CHB patients from group A exhibited VB and 4 of them (16% of the group A CHB patients) showed both VB and BB, whereas no patient from group B exhibited VB. At mo 12 of treatment, 72% of group A patients and 100% of group B were complete responders (neither VB nor BB, P = 0.04). Both serum ALT levels and serum HBV-DNA levels were significantly reduced after the 12 mo treatment period in the two groups.
Nine of 25 CHB patients (36%) and 14 of 25 (56%) from group A exhibited VB at mo 24 and 36 of LAM monotherapy, respectively. Two of 12 CHB patients (16.6%) and 3/12 (25%) from group B exhibited VB at mo 24 and 36 of treatment, respectively. Elevation of ALT levels was observed in 8/14 (57.1%) virological breakthroughers from group A, but in none of three virological breakthroughers from group B (P = 0.001), in the follow-up period. ALT values exceeding 400 IU/L were not observed in none of them. Alterations in bilirubin levels, albumin levels, prothrombin time or episodes of hepatic decompensation during breakthrough were not observed in both groups.
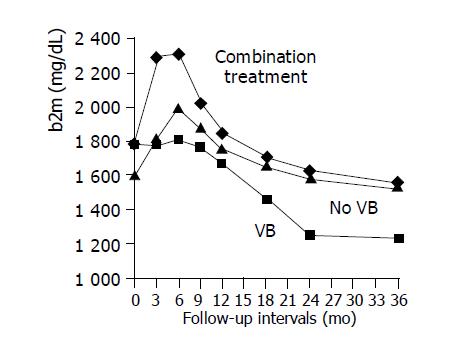
Demographics, biochemical, virological and histological information variables of patients from group A, who did (subgroup A1) and did not (subgroup A2) exhibit VB in the follow-up period, are presented in Table 2. The two subgroups were comparable for all the baseline parameters (age, ALT, HBV-DNA, grade, stage, b2m), but they differed significantly in the pattern of b2m curve during treatment. In particular, all patients who did not show VB in the follow-up period exhibited serum b2m elevation in the mo 3 of treatment compared to baseline ones, whereas only 57.1% of those with VB exhibited serum b2m elevation (P = 0.03). Moreover, the duration (in months) of serum b2m elevation was significantly longer in the responders’ subgroup of group A than the non-responders’ one (7.3±2.6 vs 3.8±3.4, P = 0.02). The different patterns of mean serum b2m levels between the treatment groups in relation to VB are presented in Figure 1. It is evident that mean serum b2m levels remain stable for about 9 mo in the subgroup of patients from group A who exhibited VB (subgroup A1), while steeply increases for about 6 mo in the subgroup of long-term LAM-treated virological responders (subgroup A2). Initial combination treatment increases significantly mean serum b2m levels during the first month of therapy in all CHB patients from group B, as shown in Table 1 and Figure 1. Subsequently, serum b2m values decline in all groups.
| Subgroup A1 (n = 14) | Subgroup A2 (n = 11) | P | |
| Age (yr) | 41.9±12.9 | 43.9±5.0 | 0.75 |
| ALT (IU/L) | 183.6±87.7 | 263.9±115.8 | 0.09 |
| HBV-DNA -log10 (copies/mL) | 6.21±0.97 | 5.63±0.56 | 0.13 |
| GRADE (0-18) | 7.1±3.3 | 6.8±3.5 | 0.84 |
| STAGE (0-6) | 3.4±1.9 | 3.1±1.3 | 0.65 |
| B2m (mg/dL) (baseline levels) | 1775.5±304.1 | 1588.8±391.6 | 0.23 |
| Duration of b2m elevation (mo) | 3.8±3.4 | 7.3±2.6 | 0.02a |
| B2m elevation in 3 mo (%) | 8/14 (57.1) | 11/11 (100) | 0.03a |
Mean baseline serum b2m levels were slightly higher in patients with high initial serum HBV-DNA compared to patients with low initial HBV-DNA (1878 mg/dL vs 1622 mg/dL; t = -1.58, P = 0.13). Patients with higher baseline serum b2m levels had slightly higher baseline HAI (r = 0.39; P = 0.08) and slightly higher baseline ALT levels (r = 0.25; P = 0.28), although non-significantly. Patients from group A with high initial serum HBV-DNA levels had shorter duration of serum b2m elevation (6.2 mo for the low vs 3 mo for the high initial HBV-DNA group; t = 1.49; P = 0.186). Moreover, it is interesting to note that the vast majority (99.3%) of the initial low HBV-DNA group of patients from group A exhibited b2m elevation at 3 mo, while only 33% of the initial high HBV-DNA group (χ2 = 8.5; P = 0.004). All patients from group B exhibited serum b2m elevation during the first months of treatment, irrespective of their initial HBV-DNA status.
Survival analyses have the advantage of combining information about both frequency of events and time of their occurrence. The reported rate ratios (RR) indicate risk of having VB for each time unit of follow-up. They therefore reflect not only how likely it is for a patient to recur but also how fast he may be expected to do so.
When the duration of serum b2m elevation was used as predictor, lower score (decreased duration of b2m elevation) was associated with an increased risk of VB (RR = 1.25; 95%CI, 1.03-1.52, P = 0.026) (Table 3). It is of importance to note here that in this model the RR reflects the increase in risk for VB for each additional unit of the predictor (i.e., each less month for which serum b2m failed to elevate). According to this result, in comparison to a patient whose serum b2m levels continued to increase for 12 mo, a patient whose b2m continued to increase only for 9 mo had 1.95 times higher risk of developing VB; a patient whose b2m continued to increase only for 6 mo had a 3.8 times higher chances of developing VB and a patient whose b2m continued to increase only for 3 mo had a 7.4 times higher risk for developing VB.
When serum b2m levels were used in its dichotomous form (reflecting elevated vs declined status at 3 mo interval), there was again an inverse association between b2m levels and VB (RR = 4.6; 95%CI, 1.22-17.36, P = 0.024) (Table 3). In other words, in comparison to patients whose serum b2m levels were increased at 3 mo, subjects whose b2m levels were decreased at 3 mo, had 4.6 times higher risk of experiencing VB.
When age, baseline ALT levels, initial serum HBV-DNA levels and grade of liver disease were simultaneously included in the same Cox model (along with b2m status at 3 mo of follow-up), decreased b2m status was still associated with increased risk of VB (RR = 12.23; 95%CI, 1.28-116.80, P = 0.03) (Table 4).
| Variable | RR | 95%CI | |
| Age (yr) | Continuous | 0.98 | 0.92–1.05 |
| ALT (IU/L) | Continuous | 0.99 | 0.98–1.00 |
| Grade (0–18) | Continuous | 1.26 | 0.98–1.63 |
| HBV-DNA (copies/mL) | High vs low | 1.94 | 0.26–14.26 |
| B2m (mg/dL) | Decline vs elevation | 12.23 | 1.28–116.801 |
In this pilot study, we found that combination (interferon plus LAM) priming followed by LAM monotherapy may delay or prevent the selection of LAM-resistance variants, compared to long-term LAM monotherapy, in HBeAg-negative CHB patients. Moreover, we observe that serum b2m levels at mo 3 of treatment, compared to baseline ones, might be a good predictor of risk for VB, a finding that needs further investigation.
The role of combination therapy in preventing VB, compared to LAM monotherapy, has been confirmed in HBeAg-negative[13,21] as well as HBeAg-positive[22] CHB patients. However, in our study the treatment period was longer enough (36 mo) in both groups of patients than other studies. This remarkable event is very important, especially in patients treated with LAM, which has a time-dependent cumulative effect in emerging HBV-DNA polymerase variants. Moreover, we used combination treatment only for the first 6 mo in the group B of our study population, followed by LAM monotherapy thereafter.
Early prognosis of VB during LAM treatment is very useful in order to predict the clinical outcome and to modify the medication. Initial serum HBV-DNA levels seem to be a good predictor of virological response[7-9]. Similarly, quantitative HBV-DNA testing during LAM treatment provides prognostic information: there is low likelihood of response in patients who remain positive at mo 3 of treatment[23]. However, HBV-DNA testing is expensive and requires high-qualified laboratories. In contrast, the calculation of serum b2m levels using the microparticle enzyme immunoassay technology is obviously a cheaper and easier method to use.
In our study, we found a significant correlation between the decline of serum b2m levels, especially during the first 3 mo of treatment, and the risk for VB in HBeAg-negative CHB patients. Moreover, we found that the longer duration of serum b2m elevation is associated with a reduced risk of VB in HBeAg-negative CHB patients under long-term LAM monotherapy. In particular we found that for each additional month where serum b2m continued to elevate, the risk for VB reduced approximately 80% (or for each less month of b2m elevation the risk increased by 1.25 times). Serum b2m elevation in the long-term LAM-treated virological responders’ subgroup, during the first months of treatment, possibly represents an elevated endogenous immune response in this group of patients. The majority of them (99.3%) had low initial serum HBV-DNA levels (≤106 copies/mL), a finding that supports this hypothesis. These patients exhibit a comparable serum b2m curve with the corresponding one of the patients from the initial combination treatment group, as shown in Figure 1.
Chronic HBV infection is characterized by T-cell hyporesponsiveness and stimulation of HBV-specific T-cell responses in patients with CHB is believed to represent a rational strategy to treat persistent infection[24]. Activation of T-cell immune responses leads to elimination of intracellular virus by cytolytic destruction of infected hepatocytes and suppression of viral gene expression, which is caused by cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-alpha (TNF-α), secreted by activated T-cells (Th1 subset) at the site of infection[25-27]. This is involved in the T-cell response providing help to cytotoxic T-lymphocytes, mediating in HBV elimination by cytolytic and non-cytolytic mechanisms[28]. Serum b2m levels are significantly elevated in patients with chronic viral hepatitis, compared to healthy controls[15,17] and increase significantly on interferon-alpha treatment[16-18], representing a Th1-dominant immune response. However, in our study serum b2m levels during the first month of treatment increase significantly, compared to baseline values, not only in the group of patients who receive initial combination treatment, but also in the virological responder subgroup of LAM-treated patients, suggesting that common antiviral and immunomodulatory mechanisms may exist between these two groups.
Serum b2m levels have been anticipated to represent the turnover of HLA antigens and are associated with lymphocyte proliferation and activation[29]. Boni et al[30], suggest that LAM treatment can overcome cytotoxic T-cell hyporesponsiveness in treated CHB patients, by reducing the levels of viremia. High viral load reduces the number and the potential activity of circulating HBV-specific T-lymphocytes compared with low viral load in CHB patients[31], suggesting the dominant role of the control of viral replication. High viral load and intrahepatic viral replication results in decreased hepatocellular presentation of class I HLA molecules and acts as a negative modulator of NK-cells activity[32]. LAM treatment reduced the intrahepatic viral load[33], resulting possibly in the modulation of T-cellular immune response. On the other hand, Marinos et al[34], suggest that the profound inhibition of HBV replication by nucleoside analogues does not restore the impaired virus-specific T-cell responses.
The association between the initial low serum HBV-DNA levels and serum b2m elevation during LAM monotherapy, observed in the first group of our study population, implies an activation of host T-cellular immune responses before the beginning of LAM treatment, in the virological responders’ subgroup, that leads to the control of viremia in the long term. The comparable baseline serum ALT levels and histological findings between the two subgroups of group A and the ALT normalization during the first months of treatment in both subgroups suggest possibly an additive Th1-mediated, non-cytolytic inhibition of virus replication in the responders subgroup, as have been shown by other studies[35], a possibility that needs further investigation. Taken together the significant elevation of serum b2m levels in all patients on initial combination (interferon plus LAM) treatment as well as in those on LAM monotherapy who responded virologically in the long-term and the strong relationship between the initial combination treatment and the prevention of VB, we suggest that close monitoring of serum b2m levels in HBeAg-negative CHB patients under LAM monotherapy could result in the early recognition of the group of breakthroughers and the possible addition of immunomodulatory and/or other antiviral drugs.
Initial serum HBV-DNA levels differed between the wo subgroups of group A patients (virological responders vs non-responders), as expected from the literature[7-9], but not significantly (Table 2). Also initial serum HBV-DNA status was not a significant predictor in the adjusted Cox model (Table 4). This may be because of decreased power of the current study due to the small number of the study population. However, serum b2m status remained significant in the prognosis of VB, in the adjusted Cox model (which was adjusting for baseline HBV-DNA status). These results suggest that there is a predictive value of serum b2m levels, compared to baseline ones, for the emergence of HBV-DNA polymerase mutants in CHB patients under long-term LAM treatment.
Limitations of our study were the lack of calculation of Th-1 (TNFα, IFNγ, etc.,) and/or Th-2 (IL-4, IL-6, etc.,) cytokines in serum of CHB patients under the long-term treatment schedules, as well as the absence of b2m staining in liver biopsy specimens.
In conclusion, in CHB patients, the initial combination treatment followed by LAM monotherapy seems to delay or prevent the emergence of HBV-DNA polymerase variants, compared to long-term LAM monotherapy, irrespective of baseline parameters. In HBeAg-negative CHB patients under either long-term LAM monotherapy or initial combination treatment, serum b2m levels at 3 mo of treatment, compared to baseline values, might be a predictor of risk for VB. Decreased serum b2m levels at mo 3 of treatment are associated with 4.6 times higher risk of VB, in comparison to patients whose serum b2m levels were increased at the same time. The shorter duration of serum b2m elevation in LAM-treated patients is associated with an increased risk of VB in the long term, suggesting frequent follow-up visits and possibly reinforcement of treatment strategies.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | The World Health Report. WHO 1998. . |
| 2. | Schalm SW, Thomas HC, Hadziyannis SJ. Chronic hepatitis B. Prog Liver Dis. 1990;9:443-462. [PubMed] |
| 3. | Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, Thomas HC. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 861] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Laras A, Koskinas J, Avgidis K, Hadziyannis SJ. Incidence and clinical significance of hepatitis B virus precore gene translation initiation mutations in e antigen-negative patients. J Viral Hepat. 1998;5:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Brunetto MR, Giarin M, Saracco G, Oliveri F, Calvo P, Capra G, Randone A, Abate ML, Manzini P, Capalbo M. Hepatitis B virus unable to secrete e antigen and response to interferon in chronic hepatitis B. Gastroenterology. 1993;105:845-850. [PubMed] |
| 6. | Hadziyannis S, Bramou T, Makris A, Moussoulis G, Zignego L, Papaioannou C. Interferon alfa-2b treatment of HBeAg negative/serum HBV DNA positive chronic active hepatitis type B. J Hepatol. 1990;11 Suppl 1:S133-S136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 8. | Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 487] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Akuta N, Suzuki F, Kobayashi M, Matsuda M, Sato J, Takagi K, Tsubota A, Suzuki Y, Hosaka T, Someya T. Virological and biochemical relapse according to YMDD motif mutant type during long-term lamivudine monotherapy. J Med Virol. 2003;71:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Papatheodoridis GV, Dimou E, Laras A, Papadimitropoulos V, Hadziyannis SJ. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology. 2002;36:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Rizzetto M. Efficacy of lamivudine in HBeAg-negative chronic hepatitis B. J Med Virol. 2002;66:435-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Santantonio T, Niro GA, Sinisi E, Leandro G, Insalata M, Guastadisegni A, Facciorusso D, Gravinese E, Andriulli A, Pastore G. Lamivudine/interferon combination therapy in anti-HBe positive chronic hepatitis B patients: a controlled pilot study. J Hepatol. 2002;36:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Malaguarnera M, Restuccia S, Di Fazio I, Zoccolo AM, Trovato BA, Pistone G. Serum beta2-microglobulin in chronic hepatitis C. Dig Dis Sci. 1997;42:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Freni MA, Ajello A, Spadaro A, Fava A, Calapristi I, Marafioti T, Alessi N, Resta ML, Ferraù O. Class I HLA antigens hepatic display and beta-2-microglobulin serum values in chronic hepatitis C: effect of treatment with recombinant alpha interferon. Hepatogastroenterology. 1997;44:1295-1301. [PubMed] |
| 16. | Quiroga JA, Martin J, Pardo M, Carreño V. Serum levels of soluble immune factors and pathogenesis of chronic hepatitis C, and their relation to therapeutic response to interferon-alpha. Dig Dis Sci. 1994;39:2485-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kumashiro R, Ide T, Sasaki M, Murashima S, Suzuki H, Hino T, Morita Y, Miyajima I, Ogata K, Tanaka E. Interferon-gamma brings additive anti-viral environment when combined with interferon-alpha in patients with chronic hepatitis C. Hepatol Res. 2002;22:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | García-Buey L, López-Botet M, García-Sánchez A, Balboa MA, Aramburu J, García-Monzón C, Acevedo A, Moreno-Otero R. Variability in the expression of a beta 2-microglobulin epitope on hepatocytes in chronic type C hepatitis on treatment with interferon. Hepatology. 1993;17:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wejstål R, Norkrans G, Weiland O, Schvarcz R, Fuchs D, Wachter H, Fryden A, Glaumann H. Lymphocyte subsets and beta 2-microglobulin expression in chronic hepatitis C/non-A, non-B. Effects of interferon-alpha treatment. Clin Exp Immunol. 1992;87:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3782] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 21. | Tatulli I, Francavilla R, Rizzo GL, Vinciguerra V, Ierardi E, Amoruso A, Panella C, Francavilla A. Lamivudine and alpha-interferon in combination long term for precore mutant chronic hepatitis B. J Hepatol. 2001;35:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, Dhillon A, Moorat A, Barber J, Gray DF. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 365] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Buti M, Sánchez F, Cotrina M, Jardi R, Rodríguez F, Esteban R, Guardia J. Quantitative hepatitis B virus DNA testing for the early prediction of the maintenance of response during lamivudine therapy in patients with chronic hepatitis B. J Infect Dis. 2001;183:1277-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1201] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 25. | Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1161] [Cited by in RCA: 1179] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 26. | Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 319] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 840] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 28. | Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348-2357. [PubMed] |
| 29. | Bjerrum OW, Bjerrum OJ, Borregaard N. Beta 2-microglobulin in neutrophils: an intragranular protein. J Immunol. 1987;138:3913-3917. [PubMed] |
| 30. | Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, Cavalli A, Urbani S, Boehme R, Panebianco R. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology. 2001;33:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 657] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 32. | Michalak TI, Hodgson PD, Churchill ND. Posttranscriptional inhibition of class I major histocompatibility complex presentation on hepatocytes and lymphoid cells in chronic woodchuck hepatitis virus infection. J Virol. 2000;74:4483-4494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Tomita T, Yokosuka O, Tagawa M, Saisho H, Tamura S, Fukuda I, Omata M. Decrease of wild-type and precore mutant duck hepatitis B virus replication during lamivudine treatment in white Pekin ducks infected with the viruses. J Hepatol. 2000;32:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Marinos G, Naoumov NV, Williams R. Impact of complete inhibition of viral replication on the cellular immune response in chronic hepatitis B virus infection. Hepatology. 1996;24:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Suri D, Schilling R, Lopes AR, Mullerova I, Colucci G, Williams R, Naoumov NV. Non-cytolytic inhibition of hepatitis B virus replication in human hepatocytes. J Hepatol. 2001;35:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |