Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1709
Revised: August 12, 2004
Accepted: October 10, 2004
Published online: March 21, 2005
AIM: To find suitable solutions having lesser granules and keeping erythrocytes in normal shapes under atomic force microscopy (AFM).
METHODS: Eight kinds of solutions, 1% formaldehyde, PBS buffer (pH7.2), citrate buffer (pH6.0), 0.9% NaCl, 5% dextrose, TAE, 1640 medium and 5% EDTA-K2, were selected from commonly used laboratory solutions, and venous blood from a healthy human volunteer was drawn and anticoagulated with EDTA-K2. Before scanned by AFM (NanoScopeIIIa SPM, Digital Instruments, Santa Barbara, CA), a kind of intermixture was deposited on freshly cleaved mica and then dried in the constant temperature cabinet (37 °C).
RESULTS: One percent formaldehyde, citrate buffer, 5% dextrose, TAE, were found to keep human erythrocytes in normal shape with few particles. Processed by these solutions, fine structures of human erythrocyte membrane were obtained.
CONCLUSION: One percent formaldehyde, citrate buffer, 5% dextrose and TAE may be applied to dispose erythrocytes in AFM. The results may offer meaningful data for clinical diagnosis of blood by AFM.
- Citation: Ji XL, Ma YM, Yin T, Shen MS, Xu X, Guan W. Application of atomic force microscopy in blood research. World J Gastroenterol 2005; 11(11): 1709-1711
- URL: https://www.wjgnet.com/1007-9327/full/v11/i11/1709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i11.1709
Human beings have experienced three revolutions in the observation of cells. Since 1665, when the first light microscopy was invented, the revolution of this kind of instruments has seen great improvements. But with the limitation of the wavelength of visible light, smaller structures of cells cannot be observed.
In the 20th century, with the development of electronics, electron microscope emerged. Though organelles could be detected under electron microscope, the samples often required special treatment, such as coating, staining and drying in vacuum. Due to these complicated preparation steps, surface information ran a risk of artifacts.
In 1986 the invention of atomic force microscopy (AFM)[1] provided a great source of information the detection of cell structures and this was a turning point. In principle, the AFM images are generated by moving a tip attached to a soft cantilever over the sample surface while recording its deflections as change of position. With atomic resolution and simple sample preparation, since the invention of atomic force microscopy (AFM) in 1986, AFM has been applied to many fields, such as physics, chemistry, biology and mechanics[2-4]. However, the application of AFM in medicine especially in clinical diagnosis is far from being developed. To dispose human cells with suitable solutions AFM is necessary in clinical diagnosis. The aim of this experiment was to find suitable solutions, which could easily be obtained, having lesser granules and keeping erythrocyte in normal shapes, under AFM. Here, eight kinds of laboratory’s common solutions were selected to dilute human erythrocytes from which we found four of them were suitable for the observation of erythrocytes under AFM. These four kinds of solutions kept human erythrocytes in normal shape and had few particles. Processed by these solutions, fine structures of membranes of human erythrocytes were also scanned.
The experiment had two parts. In the first part, eight kinds of solutions were selected from commonly used laboratory solutions. They were 1% formaldehyde, PBS buffer (pH7.2), citrate buffer (pH6.0), 0.9% NaCl, 5% dextrose, TAE, 1640 medium and 5% EDTA-K2. These solutions were separately deposited on freshly cleaved mica and then dried in a constant temperature cabinet (37 °C) for AFM observation. In the second part, venous blood from a healthy human volunteer was drawn and anticoagulated with EDTA-K2 Then normal human blood was mixed with the solutions separately and stored in a refrigerator (4 °C). Before scanning by AFM, a kind of intermixture was deposited on freshly cleaved mica, then dried in the constant temperature cabinet (37 °C).
AFM (NanoScope IIIa SPM, Digital Instruments, Santa Barbara, CA) was placed on an air-supported anti-vibration table. In the first part, commercially available contact mode cantilevers were used (Digital Instruments). Ten areas of each kind of solutions were obtained with scan area 50 μm ×50 μm and scan rate 1 Hz. In the second part, commercially available tapping mode cantilevers were used (Digital Instruments). Five areas of each sample were scanned. Each area was observed with a scan size of 30 μm, erythrocyte and 1 μm as well as 0.5 μm (0.5 μm area was the magnification of 1 μm area) in both periphery and middle of membranes of erythrocytes. Scan rate was 0.5-1.5 Hz.
In daily clinical pathological diagnosis with light microscope and electron microscope, it is very important to observe human cells. We tried to apply AFM for pathological diagnosis. Thus the observation of human cells was necessary. But in most cases human cells should be disposed by solutions. Therefore, the solutions, which have less granules[5] and could keep cells in normal shapes and diameters, were required. We selected eight kinds of solutions, which were commonly used in laboratory to be observed by AFM.
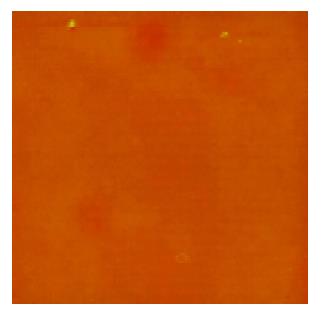
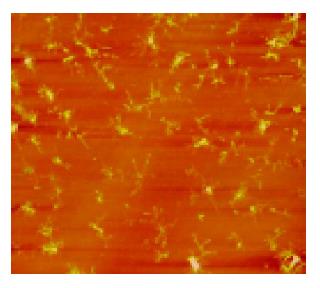
A few of irregular granules were scanned in 1% formaldehyde with sizes of 0.5-2 μm. Several irregular granules were scanned in citrate buffer with sizes of 0.5-3 μm. Some irregular granules were scanned in 5% dextrose with sizes of 0.5-4 μm (Figure 1). A few of irregular granules were scanned in TAE with sizes of 0.6-7 μm. Several irregular granules were scanned in 5% EDTA-K2 with sizes of 0.5-4 μm. A lot of granules were observed in PBS buffer with four kinds of shapes; finely branched granules with a size of 6 μm (Figure 2), bulkily branched ones with a size of 10 μm, cross ones with sizes of 3-6 μm and irregular ones with sizes of 0.3-1.5 μm. Many granules were also found in 0.9% NaCl with four kinds of shapes; butterfly granules with sizes of 0.3-7 μm, branched ones with sizes of 8-20 μm, diamond-shaped ones with a size of 24 μm and irregular ones with sizes of 0.4-10 μm. A lot of snow-shaped granules were observed in 1640 medium.
From the above data, 1% formaldehyde, citrate buffer, 5% dextrose, TAE and 5% EDTA-K2 had a few granules. These five solutions may be applied to further observations.
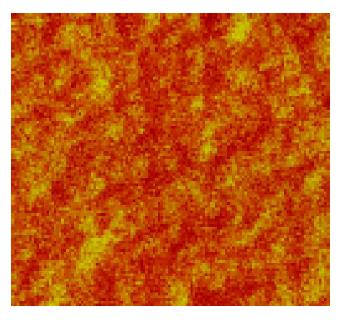
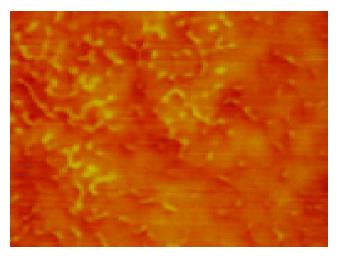
To further select the suitable solutions, which could be applied to dispose human cells for AFM observation, these eight kinds of solutions were mixed with human blood. In samples of 1% formaldehyde with blood, citrate buffer with blood, 5% dextrose with blood, and TAE with blood, the following were found; firstly, few granules existed in images; secondly, human erythrocytes were bi-concave with diameters from 7 to 8 μm (normal diameters of human erythrocytes is 7 to 8.5 μm); thirdly, fine structures of human erythrocyte membranes featured with the shape of pores (15-100 nm) and protrusions (15-100 nm), the protrusions were around the pores, and the protrusions and the pores were in a waffle-like pattern (Figure 3). In terms of sizes and arrangements of pores and protrusions, there were no differences between the periphery and the middle of erythrocyte membranes. However, in samples of PBS buffer with blood (Figure 4), 0.9% NaCl with blood, 1640 medium with blood, and 5% EDTA-K2 with blood, a lot of granules existed in images, which were interferences in the observation. So these four solutions were abandoned. Human blood was also mixed separately with 5% formaldehyde, 3% glutaraldehyde and Tris buffer (0.05 mol/L, pH7.2). In 5% formaldehyde, the diameters of human erythrocytes were about 6 μm, which were smaller than normal ones. Three percent glutaraldehyde did not make the erythrocytes scatter evenly, and at the same time this kind of solution is poisonous. Tris buffer was mixed with human blood with the phenomena of hemolysis. Thus 5% formaldehyde, 3% glutaraldehyde and Tris buffer were also abandoned. From the above, 1% formaldehyde, citrate buffer, 5% dextrose and TAE are suitable for observation of human erythrocytes under AFM.
The lipid-globular protein mosaic model (LGPM) was developed by Singer and Nicolson in 1972[6]. They introduced that integral proteins and peripheral proteins are associated with lipid bilayer membranes. The pores and the protrusions observed in fine structures of human erythrocyte membranes might reflect the LGPM model.
As is known, there are several forms of transmembrane transport. In facilitated diffusion, substances with little solubility are transferred by special proteins. There are two types of facilitated diffusion. The first one is conducted by carriers which take dextroses, amino acids and mesostates through. The second one is carried out by channels, which let Na+, K+, Ca2+, Cl- through. The pores and the protrusions observed in fine structures of human erythrocyte membranes might be these special proteins of facilitated diffusion especially the pores were very similar with the channels. In active transport, the most famous is Na+-K+ pumps, which are special proteins embedded in membrane lipid bilayers. This kind of pumps transfers Na+ and K+ with consumption of ATP. With the methods of molecular biology, it is found that Na+-K+ pump is made up of α subunit and β subunit. The pores and the protrusions observed in fine structures of human erythrocyte membranes might be Na+-K+ pumps.
Receptors are very important in membranes, which are associated with many kinds of functions. In the surface of erythrocytes, there exist blood group antigens. The fine structures of human erythrocyte membranes probably contained receptors, for example, transferrin, and blood group antigens.
In terms of transmembrane signaling, voltage-gated channel especially Na+ channels have been well studied. Na+ channels consist of three subunits - α peptide chain, β1 peptide chain and β2 peptide chain, while their functions are really done by α peptide chain. α subunit contains four similar domains, and each domain has six transmembrane α-spirals, which are made up of hydrophobic amino acids. These four domains and hydrophobic α-spirals form a channel-like structure. When transmembrane potential is changed, these α-spirals move their sites because of their own electron charges; therefore, the “gates” of channels are opened. Other studies suggest that the second and the third α-spirals form the “inner wall” of the channel. The channel features are consistent with what had been observed in fine structures of human erythrocyte membranes.
Many kinds of diseases are pathological changes of proteins, which are difficult to be detected by light microscopy and electron microscopy. However, with the advantage of high resolution, the emergence of AFM makes it easy. By AFM the lesions of proteins could be distinctly observed. Therefore, AFM will be a very important instrument in daily diagnosis. Notwithstanding some methods of disposing erythrocytes[7-9], which are complicated and many of them follow the ways of electron microscope, in this experiment, a new way of disposing human cells was found for pathological diagnosis with AFM. Four kinds of suitable solutions were selected and the fine structures of human erythrocyte membranes observed might form the basis of further studies and diagnosis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56:930-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11356] [Cited by in RCA: 5355] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 2. | Blank S, Arnoldi M, Khoshnavaz S, Treccani L, Kuntz M, Mann K, Grathwohl G, Fritz M. The nacre protein perlucin nucleates growth of calcium carbonate crystals. J Microsc. 2003;212:280-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Janićijević A, Ristic D, Wyman C. The molecular machines of DNA repair: scanning force microscopy analysis of their architecture. J Microsc. 2003;212:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ji X, Oh J, Dunker AK, Hipps KW. Effects of relative humidity and applied force on atomic force microscopy images of the filamentous phage fd. Ultramicroscopy. 1998;72:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Ma YM, Ji XL, Yin T, Xu X, Shen MS. Nine kinds of common liquids observed by atomic force microscopy. Zhongguo Shiyan Zhenduanxue. 2004;8:38-39. |
| 6. | Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6562] [Cited by in RCA: 5427] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 7. | Zhang PC, Bai C, Huang YM, Zhao H, Fang Y, Wang NX, Li Q. Atomic force microscopy study of fine structures of the entire surface of red blood cells. Scanning Microsc. 1995;9:981-989; discussion 1009-1010. [PubMed] |
| 8. | Zachée P, Snauwaert J, Vandenberghe P, Hellemans L, Boogaerts M. Imaging red blood cells with the atomic force microscope. Br J Haematol. 1996;95:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Zachée P, Boogaerts M, Snauwaert J, Hellemans L. Imaging uremic red blood cells with the atomic force microscope. Am J Nephrol. 1994;14:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |