Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1685
Revised: September 1, 2004
Accepted: September 3, 2004
Published online: March 21, 2005
AIM: To study the interactive relationship of gallbladder motor function, plasma cholecystokinin (CCK) and cholecystokinin A receptor (CCK-R) of gallbladder in patients with cholesterol stone disease.
METHODS: Gallbladder motility was studied by ultrasonography in 33 patients with gallbladder stone and 10 health subjects as controls. Plasma CCK concentration was measured by radioimmunoassay in fasting status (CCK-f) and in 30 min after lipid test meal (CCK-30). Radioligand method was employed to analyze the amount and activity of CCK-R from 33 gallstone patients having cholecystectomy and 8 persons without gallstone died of severe trauma as controls.
RESULTS: The percentage of cholesterol in the gallstone composition was more than 70%. The cholesterol stone type was indicated for the patients with gallbladder stone in this study. Based on the criterion of gallbladder residual fraction of the control group, 33 gallstone patients were divided into two subgroups, contractor group (14 cases) and non-contractor group (19 cases). The concentration of CCK-30 was significantly higher in non-contractor group than that in both contractor group and control group (55.86±3.86 pmol/L vs 37.85±0.88 pmol/L and 37.95±0.74 pmol/L, P<0.01), but there was no difference between contractor group and control group. Meanwhile no significant difference of the concentration of CCK-f could be observed among three groups. The amount of CCK-R was lower in non-contractor group than those in both control group and contractor group (10.27±0.94 fmol/mg vs 24.59±2.39 fmol/mg and 22.66±0.55 fmol/mg, P<0.01). The activity of CCK-R shown as KD in non-contractor group decreased compared to that in control group and contractor group. Only was the activity of CCK-R lower in contractor group than that in control group. The ejection fraction correlated closely with the amount of CCK-R (r = 0.9683, P<0.01), and the concentration of CCK-30 correlated negatively with the amount of CCK-R closely (r = -0.9627, P<0.01).
CONCLUSION: The distinctive interactive relationship of gallbladder emptying, plasma CCK and CCK-R in gallbladder from this study suggested that the defect of CCK-R may be a key point leading to the impairment of gallbladder motor function and the pathogenesis of cholesterol gallstone formation may differ in two subgroups of gallstone patient, gallbladder non-contractor group or contractor group.
- Citation: Zhu J, Han TQ, Chen S, Jiang Y, Zhang SD. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol 2005; 11(11): 1685-1689
- URL: https://www.wjgnet.com/1007-9327/full/v11/i11/1685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i11.1685
Gallbladder motility is regulated by cholinergic system and gastrointestinal hormone[1,2]. Postprandial gallbladder emptying is triggered mainly by plasma cholecystokinin (CCK) from small intestine. CCK interacts with CCK receptor-A (CCK-R) in gallbladder, which in turn elicits the contraction of gallbladder by the activation of post-membrane signaling passage in smooth muscle[3].
Abnormal gallbladder emptying may play an important role in cholesterol gallstone formation[2,4]. Recent studies have revealed that patients having gallbladder stone can be divided into two subgroups with regard to gallbladder emptying: normal contractor and pathological contractor. The two subgroups may differ in the pathogenesis of cholesterol stone formation[5,6]. The purpose of this study was to examine if there is a difference in gallbladder motor function, plasma CCK concentration, the amount and activity of CCK-R in the two subgroups, and to identify the role in pathogenesis of cholesterol gallstone formation.
Thirty-three patients with gallbladder stone (16 male and 17 female, median age 48.3±10.8 years) and 10 controls (4 male and 6 female, age 38.7±9.0 years) were enrolled into this study. Thirteen of gallstone patients suffered from episodes of biliary colic one month ago, and the others had vague right upper quadrant pain. Gallstone volume was no more than one-third of gallbladder volume as assessed by ultrasonography. None of subjects had diabetes mellitus, history of diseases or operations to affect gallbladder motility. None received any medication such as cholic acid and somatostatin to influence gallbladder motor function in recent times. All of subjects had normal gallbladder wall (no more than 2 mm), common bile duct by ultrasound study, and normal liver function by blood biochemistry test. With the analysis of gallstone composition, more than 70% cholesterol was shown in the gallstones from cholecystectomy in 33 patients. Control group for CCK-R measurement was composed of 8 persons (7 males and 1 female) without gallstone died of severe trauma. All samples were maintained in a highly controlled manner, insuring patient confidentiality.
Gallbladder motor function Gallbladder volumes were measured sonographically by Aloka 650 B-type ultrasonograph equipped with a transducer 3.5 mHz. The calculation was performed according to the sum of cylinders method[7,8]. The subjects in two groups were studied after an overnight fast. Fast volume (FV) was measured before the liquid test meal (500 mL, fat 25 g, protein 40 g and carbohydrates 65 g). The postprandial gallbladder volume was recorded 30, 60 and 90 min after the liquid test meal. Residual volume (RV) was defined as the smallest postprandial volume. Residual fraction (RF) was calculated with the formula (RV/FV×100%) and ejection fraction (EF) with the formula ((FV-RV)/FV×100%)[9].
Plasma CCK concentration measurements Venous blood samples were collected in iced tubes containing heparin for plasma CCK determination before the test meal and 30 min afterwards. Aprotinin (Sigma, Co.) was added immediately. After centrifugation (3000 r/min, 15 min), plasma was frozen at -20 °C until a specific radioimmunoassay for plasma CCK[10]. We measured postprandial CCK concentration at 30 min after test meal in view of the fact that plasma CCK concentration reaches peak and keeps constant for a short period of time[10]. Both fasting plasma CCK concentration (CCK-f) and CCK concentration at 30 min after test meal (CCK-30) were taken into account in the study.
CCK-R concentration measurements Gallbladder samples were collected from the 33 gallbladder stone patients having cholecystectomy and 8 control subjects without gallstone. Tissue samples were snap-frozen in liquid nitrogen immediately. CCK-R was determined by radioligand blinding assay as described previously[11,12]. 125I-BH-CCK8 (Amersham, Co., radioactivity 2000 Ci/moL) was used as the specific radioligand and CCK8 (Sigma, Co.) as the non-radioactive ligand for competition. The activity of CCK-R was defined as KD (Equilibrium Association Constant), which meant the affinity between CCK and CCK-R. The lower KD of CCK-R suggested the higher activity[12,13].
Results were expressed as the mean±SE. The mean and slope of regression were evaluated by the Student’s t-test. The relationship between two groups of variants was examined by linear regression analysis (Pearson’s correlation coefficient), and a P value less than 0.05 was considered statistically significant.
According to Pomeranz and Shaffer, pathological contractor patients (non-contractor) were defined by a RF exceeding the mean of controls added 2 SD[5]. In this study, patients having gallbladder stone were divided into two subgroups: gallbladder contractor (14 subjects) and non-contractor (19 subjects).
Not only the patients in non-contractor group but also those in contractor group exhibited a significantly enlarged RV than in control group and the patients in non-contractor group had a markedly enlarged RV than in contractor group (P<0.01). FV in patients of contractor group was distinctly large than that in control (P<0.05); however, it did not show any significant difference between contractor group and non-contractor group, as well as between control group and non-contractor group (Table 1).
There was no difference of CCK-f in plasma among three groups. Plasma CCK-30 of patients in non-contractor group was much higher than in contractor group and control group (P<0.01). But no difference could be identified in CCK-30 between contractor group and control group (Table 2).
The amount and activity of gallbladder CCK-R from three groups was summarized in Table 2. The amount of CCK-R in non-contractor group was significantly lower than that in contractor group and control group (P<0.01). The amount of CCK-R in contractor group tended to decrease slightly compared to that in control group despite of no significant difference. The activity of CCK-R (shown as KD of CCK-R) in gallstone patients of two groups was much lower than that in control group (P<0.01). In addition, the activity of CCK-R in non-contractor group decreased markedly than that in contractor group (P<0.01).
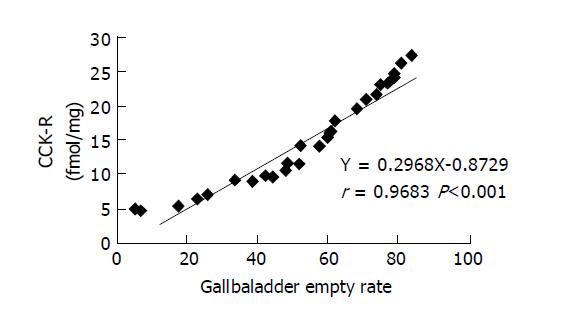
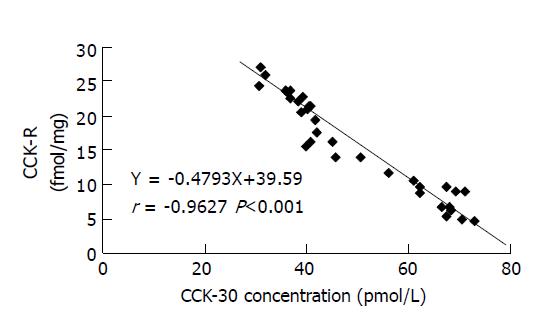
The relationship between amount of CCK-R of gallbladder and EF of gallbladder in gallstone patients was shown in Figure 1. Figure 2 showed the amount of CCK-R of gallbladder and CCK-30 concentration. EF of gallbladder correlated with the amount of CCK-R of gallbladder closely in 33 gallstone patients with positive coefficient of correlation of 0.9683, P<0.01. The concentration of CCK-30 in plasma also showed a close correlation with the amount of CCK-R of gallbladder in gallstone patients but with negative coefficient of correlation of -0.9627, P<0.01. We did not include 8 control subjects in the correlation analysis for CCK-R measurements because we could not measure their gallbladder motor function and CCK concentrations in plasma.
The purpose of this study was to explore the possible role of gallbladder in cholesterol stone formation. The analyses of gallstone composition confirmed the patients in this study suffering from the cholesterol gallstone disease. Impaired gallbladder motility might be one of the key determinants of cholesterol stone formation[2,4]. It has been reported that the process of plasma CCK binding its receptor in gallbladder plays an important role in the regulation of gallbladder emptying, but the results from recent studies were still controversial[14]. The reason may result from the evidence that two modes of gallbladder motility exist in gallstone patients, and the change in CCK and CCK-R may differ from each other. Our study confirmed that there were two groups of cholesterol gallstone patients, gallbladder contractor group and non-contractor group. Gallbladder motor function of the patients in the two groups will be analyzed. The association of gallbladder motility with CCK in plasma and CCK-R of gallbladder will be focused as following.
Patients in both contractor group and non-contractor group had an enlarged RV than control subjects. The enlarged RV induces the retention of bile in gallbladder, which may facilitate the nucleation of cholesterol crystals and stone growth in gallbladder. The enlarged RV might be the final consequence of gallbladder motility[6,9]. The mechanism of the enlarged RV may be different in two gallstone subgroups. A markedly increased FV was found in patients of contractor group compared to that in control group. There was an increased RV in patients of contractor group as a result of EF unchanged. A lower EF in patients of non-contractor group would result in RV increased, which induced bile stasis in spite of FV of gallbladder similar to that in control. So the reason of enlarged RV was an increased FV for the patients of contractor group and a lower EF for the patients of non-contractor group. It can be speculated from this study that the difference in gallbladder motility between two gallstone subgroups might result from the alteration of plasma CCK and CCK-R of gallbladder.
As shown in Table 2, the activity of gallbladder CCK-R in patients of contractor group decreased with stable amount of CCK-R and the postprandial concentration of CCK-30. It was inferred that the postprandial EF would enhance to compensate for increment in FV following the increase of the CCK-R activity. The RV of gallbladder in patients of contractor group would be close to the RV of control group, which leads to excreting the surplus of bile. Meanwhile, the decreased activity of CCK-R could be responsible for an abnormally increased FV in patients of contractor group. Gallbladder tension at rest controls the FV of gallbladder and FV may increase abnormally based on the gallbladder hypotonus. The gallbladder tension is maintained by myogenic tonus depending on some humoral and neural transmitters such as CCK and acetylcholine[1,4]. The decrease of CCK-R activity of gallbladder may induce its low affinity to plasma CCK, which contributes to gallbladder hypotonus[9,13]. Abnormally increased FV could be a risk factor of gallstone formation and an early indicator of prognosis of gallstone disease[9,15,16].
Not only the activity but also the amount of CCK-R decreased to a great extent in patients of non-contractor subgroup (Table 2). It was evident that gallbladder hypomotility in non-contractor group results from the defect of CCK-R of gallbladder[11,17,18]. On the contrary, postprandial CCK concentration (shown as CCK-30 in Table 2) of non-contractor group increased significantly compared to that in contractor group and control group, which is considered to be receptor resistance. This phenomenon could be also related with the defect of CCK-R of gallbladder[1,6]. Plasma CCK concentration may reach the peak at a specific postprandial time and keep constant for a period of time, which is important in the maintenance of continuous gallbladder contraction[10]. We speculated that there may be a dynamic equilibrium between the measurable plasma CCK and the CCK combined with CCK-R of gallbladder at the specific postprandial time and they would transform each other. A decreased amount of CCK-R in gallbladder led to the decreased combination of CCK, which could contribute to the measurable plasma CCK increasing. Therefore, a decreased amount of CCK-R of gallbladder should be responsible for an abnormally increased concentration of postprandial plasma CCK in patients of non-contractor group. This result was similar to that in a recent research, which showed postprandial plasma CCK concentration increased significantly in patients with cholecystectomy[19].
Our result confirmed that the EF of postprandial gallbladder correlated closely with the amount of CCK-R in gallbladder. The result was similar to that in recent studies[11,13,17], which also showed that the impaired gallbladder motility could result from the defect of CCK-R of gallbladder. The cytobiological studies on gallbladder smooth muscle cells also supported the fact that gallbladder hypomotility results from the decreased amount and activity of CCK-R in gallbladder. The conclusion may be traced back to the post-membrane message passage, which induces the contraction of gallbladder smooth muscle cells, since the intact number and function of the second messages (inositol trisphosphate and diacylglycerol), Ca++ associated proteins and G proteins[20-22].
A negative linear correlation between postprandial plasma CCK (CCK-30) and CCK-R of gallbladder was found and shown in Figure 2. This result could be due to the mechanism of receptor resistance[1,6]. The other possible reasons of negative correlation between CCK-30 and CCK-R might include the physiologic or physiochemical property of postprandial CCK concentration in plasma and the combination of CCK with CCK-R, such as saturability, reversibility and specificity[1,4]. Based on the negative correlation between CCK-30 and CCK-R it may be feasible to estimate the amount of CCK-R in gallbladder by measuring postprandial CCK concentration in plasma, which would be helpful to evaluate gallbladder motor function.
In conclusion, the impaired gallbladder emptying was identified in patients having cholesterol stone, which might result from the defect of CCK-R of gallbladder. The different mode of gallbladder emptying in cholesterol stone patients was associated with the alterations of CCK-R of gallbladder. In patients of contractor group, the activity of CCK-R decreased predominantly accompanied by an abnormally increased FV. As to the patients of non-contractor group, the activity and amount of CCK-R decreased simultaneously, with higher postprandial plasma CCK concentration. The results of our study demonstrated that the pathogenesis of gallstone formation could be quite different in the two subgroups of cholesterol stone patients. As to the clinical treatment for these two different groups of patients without symptoms, conservative therapy is suitable only for patients of contractor group. If gallstone patients with gallbladder non-contractor have conservative therapies, they are prone to have gallstone recurrence. Symptomatic patients of gallstones need cholecystectomy, whether their gallbladders are contractive or non-contractive.
Our study confirmed that there were two groups of cholesterol gallstone patients, gallbladder contractor group and non-contractor group. Gallbladder motor function of the patients in two groups will be analyzed. The association of gallbladder motility with CCK in plasma and CCK-R of gallbladder will be focused as following.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Mawe GM. Nerves and Hormones Interact to Control Gallbladder Function. News Physiol Sci. 1998;13:84-90. [PubMed] |
| 2. | Patankar R, Ozmen MM, Bailey IS, Johnson CD. Gallbladder motility, gallstones, and the surgeon. Dig Dis Sci. 1995;40:2323-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Yu P, De Petris G, Biancani P, Amaral J, Behar J. Cholecystokinin-coupled intracellular signaling in human gallbladder muscle. Gastroenterology. 1994;106:763-770. [PubMed] |
| 4. | Tierney S, Pitt HA, Lillemoe KD. Physiology and pathophysiology of gallbladder motility. Surg Clin North Am. 1993;73:1267-1290. [PubMed] |
| 5. | Pomeranz IS, Shaffer EA. Abnormal gallbladder emptying in a subgroup of patients with gallstones. Gastroenterology. 1985;88:787-791. [PubMed] |
| 6. | van Erpecum KJ, van Berge Henegouwen GP, Stolk MF, Hopman WP, Jansen JB, Lamers CB. Fasting gallbladder volume, postprandial emptying and cholecystokinin release in gallstone patients and normal subjects. J Hepatol. 1992;14:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Portincasa P, Moschetta A, Colecchia A, Festi D, Palasciano G. Measurements of gallbladder motor function by ultrasonography: towards standardization. Dig Liver Dis. 2003;35 Suppl 3:S56-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Agarwal M, Agarwal AK, Singh S, Shukla VK. An ultrasonographic evaluation of gallbladder emptying in patients with cholelithiasis. J Clin Gastroenterol. 2000;31:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Pauletzki J, Cicala M, Holl J, Sauerbruch T, Schafmayer A, Paumgartner G. Correlation between gall bladder fasting volume and postprandial emptying in patients with gall stones and healthy controls. Gut. 1993;34:1443-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Masclee AA, Jansen JB, Driessen WM, Geuskens LM, Lamers CB. Plasma cholecystokinin and gallbladder responses to intraduodenal fat in gallstone patients. Dig Dis Sci. 1989;34:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Xiao ZL, Chen Q, Amaral J, Biancani P, Jensen RT, Behar J. CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol. 1999;276:G1401-G1407. [PubMed] |
| 12. | Upp JR, Nealon WH, Singh P, Fagan CJ, Jonas AS, Greeley GH, Thompson JC. Correlation of cholecystokinin receptors with gallbladder contractility in patients with gallstones. Ann Surg. 1987;205:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Poston GJ, Singh P, Draviam E, Yao CZ, Gomez G, Thompson JC. Early stages of gallstone formation in guinea pig are associated with decreased biliary sensitivity to cholecystokinin. Dig Dis Sci. 1992;37:1236-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Glasbrenner B, Domínguez-Muñoz JE, Nelson DK, Pieramico O, Holzwarth C, Riepl RL, Malfertheiner P. Postprandial release of cholecystokinin and pancreatic polypeptide in health and in gallstone disease: relationships with gallbladder contraction. Am J Gastroenterol. 1994;89:404-410. [PubMed] |
| 15. | Petroni ML. Review article: gall-bladder motor function in obesity. Aliment Pharmacol Ther. 2000;14 Suppl 2:48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Vezina WC, Paradis RL, Grace DM, Zimmer RA, Lamont DD, Rycroft KM, King ME, Hutton LC, Chey WY. Increased volume and decreased emptying of the gallbladder in large (morbidly obese, tall normal, and muscular normal) people. Gastroenterology. 1990;98:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Mansour A, Dawoud I, Gad-El-Hak N. The potential site of disordered gallbladder contractility during the early stage of cholesterol gallstone formation. Hepatogastroenterology. 1998;45:1404-1409. [PubMed] |
| 18. | Xu QW, Shaffer EA. The potential site of impaired gallbladder contractility in an animal model of cholesterol gallstone disease. Gastroenterology. 1996;110:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | McDonnell CO, Bailey I, Stumpf T, Walsh TN, Johnson CD. The effect of cholecystectomy on plasma cholecystokinin. Am J Gastroenterol. 2002;97:2189-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Yu P, Chen Q, Harnett KM, Amaral J, Biancani P, Behar J. Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am J Physiol. 1995;269:G659-G665. [PubMed] |
| 21. | Behar J, Rhim BY, Thompson W, Biancani P. Inositol trisphosphate restores impaired human gallbladder motility associated with cholesterol stones. Gastroenterology. 1993;104:563-568. [PubMed] |
| 22. | Chen Q, Amaral J, Biancani P, Behar J. Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology. 1999;116:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |