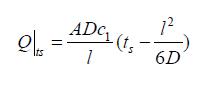Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1599
Revised: September 25, 2004
Accepted: October 18, 2004
Published online: March 21, 2005
AIM: To investigate diffusion in mammalian cell culture by gel entrapment within hollow fibers.
METHODS: Freshly isolated rat hepatocytes or human oral epidermoid carcinoma (KB) cells were entrapped in type I collagen solutions and statically cultured inside microporous and ultrafiltration hollow fibers. During the culture time collagen gel contraction, cell viability and specific function were assessed. Effective diffusion coefficients of glucose in cell-matrix gels were determined by lag time analysis in a diffusion cell.
RESULTS: Significant gel contractions occurred in the collagen gels by entrapment of either viable hepatocytes or KB cells. And the gel contraction caused a significant reduction on effective diffusion coefficient of glucose. The cell viability assay of both hepatocytes and KB cells statically cultured in hollow fibers by collagen entrapment further confirmed the existence of the inhibited mass transfer by diffusion. Urea was secreted about 50% more by hepatocytes entrapped in hollow fibers with pore size of 0.1 µm than that in hollow fibers with MWCO of 100 ku.
CONCLUSION: Cell-matrix gel and membrane pore size are the two factors relevant to the limited mass transfer by diffusion in such gel entrapment of mammalian cell culture.
- Citation: Wu DQ, Zhang GL, Shen C, Zhao Q, Li H, Meng Q. Evaluation of diffusion in gel entrapment cell culture within hollow fibers. World J Gastroenterol 2005; 11(11): 1599-1604
- URL: https://www.wjgnet.com/1007-9327/full/v11/i11/1599.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i11.1599
Entrapment of mammalian cells inside semi permeable hollow fibers has been introduced as a commonly used technique[1-5]. Based on this technique, more tissue-like culture of cells by entrapped into gel within hollow fibers have been explored as novel applications in tissue engineering[6-9], toxicology[10] and pharmacology[11,12]. Such system is comprised of two major compartments: hollow fiber membrane and gel-cell matrix, which is typically featured by ultrafiltration hollow fiber membranes and collagen gels, respectively[13]. And mass transport across hollow fibers has been well studied by taking consideration of convection together with diffusion[14-18]. When hollow fiber bioreactor is operated at low flow rate, especially in medical-related field, the primary mass transfer mechanism should be the diffusion[14], where the solute effective diffusion coefficient, representative of diffusion ability, across membranes is proportional to the transmembrane concentration gradient and decreases with the solute to pore radius ratio[19]. However, even at a very slow flow rate, convection cannot be completely negligible. Also, the diffusion across cell-collagen gel is rarely known including the extent of gel contraction and its effect on the diffusion. These were drawbacks in evaluation of mass transfer and thus hinder its further exploration as a practical technology, for an example, the clinical application of bioartificial liver in the near future. Therefore, discrete evaluation of diffusion in either membrane side or gel side should be urgently required.
Our previous research found that the liver-specific functions were much higher in the hollow fiber bioreactor featured by microporous hollow fibers with membrane pore size of 0.1 µm than in that featured by ultrafiltration hollow fibers with MWCO of 100 ku, an equivalent pore size of 0.01 µm[20]. In those bioartificial livers, hepatocytes were entrapped into collagen gels in the lumen side of the hollow fiber bioreactors with each medium circulated in either lumen or shell side. But it is uncertain that the increase of liver-specific functions is related to the better diffusion caused by bigger membrane pore size in microporous hollow fibers, because uneven distribution of fluid flow and specific configuration of hollow fibers may cause additional convective flows[21]. It seems difficult to uncouple diffusion from convection in such device of hollow fiber bioreactor. Also we never evaluated the diffusion within collagen gels.
In this paper, we evaluated the effect of diffusion by static cell culture, where solute mainly transfers by diffusion in two serial processes: membrane and cell-collagen gel. The effective diffusivities were measured, testing the diffusion ability across collagen gel with or without cells. And both the viability and specific function of collagen-entrapped cells within simple hollow fibers were examined for further evaluation of diffusion ability.
Hepatocytes from 4-6 wk old male Sprague-Dawley rats weighting 200-250 g were harvested by a two-step collagenase perfusion technique modified from that of Seglen[22]. Post-harvest viability was at least 85% by trypan blue exclusion. Hepatocytes were cultured in a basal medium of Williams’ E (Gibco) complemented with 100 U/mL penicillin and 100 U/mL streptomycin, 5 g/L bovine serum albumin (Amresco, 0530S03) and 5% fetal calf serum (Hangzhou Sijiqing Biological Eng. Material Co., Ltd., China).
Freshly harvested hepatocytes were mixed with the collagen solution, a 3:1 (v/v) mixture of type I collagen (3 g/L, prepared from rat tails in our own lab) and 4 fold concentrated Williams’ E medium with pH adjusted to 7.4 with 1 mol/L NaOH. The cell suspension with density of 1×106 cells/mL was loaded into the lumen of the long fibers with length of 80 cm. Hollow fibers were circuitously put into 12 cm dishes and maintained in a 50 mL/L CO2 incubator for collagen gelation. And 20 min later, hollow fibers were cut into equal pieces of 4 cm and immersed into 5 mL prewarmed culture medium in 60 mm culture dishes. These dishes were put back into the incubator for cell culture.
Samples for analysis of urea secretion were taken at regular intervals from the medium after mild mixing. Urea assays were performed using a Urea Nitrogen Kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
KB cells, kindly obtained from biomedical engineering institute in Zhejiang University, were cultivated in RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 U/mL streptomycin and 10% new born calf serum (Hangzhou Sijiqing Biological Eng. Material Co., Ltd., China). KB Cells was subcultured in T-flasks at a density of 105 cells at 37 °C in a 50 mL/L CO2 incubator with the medium changed every two days. Later, KB cells were detached from T-flasks by 0.5% trypin and entrapped into collagen gel within hollow fiber at the density of 4×105 cells/mL, following the same procedure as hepatocyte entrapment.
Cylindrical collagen gels were used to examine the gel contraction by both hepatocytes and KB cells in static culture. At different culture time, cylindrical collagen gels were carefully pushed out from the hollow fibers by injection with medium and suspended in the plates containing prewarmed medium. The gel diameter was then determined with a vernier eyepiece equipped on an inverted microscope.
Hepatocytes cells or KB entrapped within collagen gels were stained twice with fluorescein diacetate (FDA) and ethidium bromide (EB)[23,24]. Cylindrical collagen gels drawn from hollow fibers were stained with 2 mL of a mixture of 10 g/mL FDA and 40 g/mL EB. After 2 min incubation at room temperature followed by three rinses with PBS, the sample was examined with an epifluorescence inverted microscope. The excitation wavelength was 488 nm and the emission wavelength was 600 nm for EB and 530 nm for FDA. Cell viability was assessed by counting the number of FDA- and EB-stained cells.
A modified diffusion cell described by Hannoun and Stephanopoulos[25] was used to measure the effective diffusion coefficient of glucose in collagen gel. A plexiglass ring, with outer diameter of 8.05 cm and inner diameter of 4.55 cm, was attached by a plastic mesh at bottom to hold the covered collagen gel. Solution of 2.25 g/L collagen at pH 7, a mixture of 3 g/L type I rat tail collagen and 4 fold concentrated PBS (3:1, v/v), was immediately poured on the plexiglass ring. After 30-40 min incubation at 37 °C, collagen was gelatinized and its thickness was measured by micrometer. Then, the plexiglass covered with collagen gel was put into the diffusion cell where the upper chamber and the lower chamber were respectively 286 and 227.5 mL. The well mixing in two chambers was achieved by stirring at about 60 r/min with a magnetically driven bar in the lower cell and a stirrer in the upper cell at room temperature (21-22 °C). By taking samples in the upper chamber, the transferred glucose concentrations were determined by means of 3,5-dinitrosalicylic (DNS) colorimetric determination[26].
The corresponding diffusion coefficients were calculated according to lag-time analysis[27-29]. Assuming that there was no film mass transfer resistance, the solute concentration in the lower chamber (c1) and the solute concentration in the upper chamber (c2) can be defined as follows:
c = c1 at x = 0
c = c2 at x = l
c = 0 0≤x≤l at t = 0
Thus, the total amount Q of solute transferred through the membrane was given by the equation shown in Math 1:
Math 1
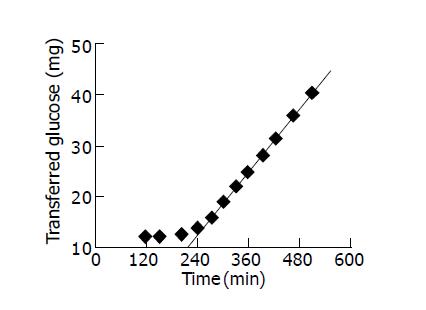
where l was the membrane thickness, A the membrane area, D the effective diffusion coefficients and ts the lag time. Figure 1 was the experimental graph of transferred glucose versus diffusion time for the diffusion of glucose. The intercept of the linear part of the curve was the so-called lag time.
To entrap hepatocytes into collagen gels, 2 mL of fresh hepatocyte slurry at density of 3×107 cells/mL was mixed with 10 mL solution of 2.7 g/L collagen to obtain a final suspension with cell density of 5×106 cells/mL and collagen concentration of 2.25 g/L. In preparation of collagen gels entrapped with dead cells, gel-cell matrix formed above was immediately deactivated by exposure to ultraviolet for 10 min. While in preparing collagen gel entrapped with viable cells, the cell-collagen matrix was cultivated in a basal medium of Williams’ E at a 50 mL/L CO2 incubator for 24 h to develop gel contraction before it was deactivated by 10 min UV radiation for effective diffusion coefficient measurement. Membranes with cell density of 0.5×106 cells/mL were similarly prepared.
Microporous polysulfone hollow fibers with average pore size of about 0.01 µm and ultrafiltration polysulfone hollow fibers with MWCO of 30 and 100 ku were purchased from Yuandong Pharmaceutical Machinery Corporation (Shanghai, China). All these hollow fibers with the same outer diameter of 1 mm, inner diameter of 0.7 mm and membrane porosity of about 90% had identical diffusion area.
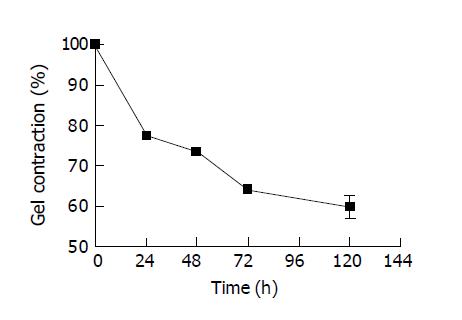
Collagen contraction was frequently reported in hepatocyte culture by collagen gel entrapment, but its contraction extent was usually determined in disc collagen gel[7]. Also, the gel contraction was rarely reported in the continuous cell culture. Hereby, the contraction of collagen gel mediated by both primary hepatocyte and KB cells were first determined in this paper by measuring the diameter of cylindrical collagen gels over the culture period of five days. The entrapped cell density of hepatocytes was set at 1×106 cells/mL with the initial gel diameter of 0.68 mm. Figure 2 demonstrates the average percentage of diameter of 3 cylindrical gels entrapped with hepatocytes to the initial gel diameter. The gel diameter rapidly decreased almost to 60% of the initial diameter within three days cultivation, while the control gel containing no cells showed no signs of contraction. At a higher cell density of 5×106 cells/mL, cell-matrix gel contracted by 60% after one day cultivation but were stable since then (data is not shown here). This was possibly due to cell death because the rapidly decreased cell viability was suspected according to the prevalent morphological change since the culture time of one day with a microscope. To confirm that dead cells caused no gel contraction, we repeated the gel contraction experiment with dead cells killed by UV for 10 min. It was found that no sign of gel contraction was observed when dead cells were entrapped with collagen gels.
Another anchorage-dependent KB cells were also randomly used to confirm gel contraction caused by cell entrapment. The initial entrapped KB cell density was set at 0.5×106 cells/mL. Compared with the initial value of 0.68 mm, the gel diameter decreased by about 14 % at 48 h and 30% at 120 h with a high cell density of around 1×107 cells/mL due to KB cell propagation. It seems primary cells are more likely to cause the gel contraction than continuous cells.
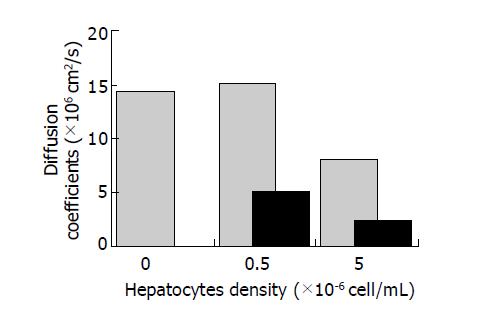
Effective diffusivities of glucose were determined in collagen gels with or without cells. Glucose was commonly selected as a marker for chemical substances involved in cell metabolisms and hepatocytes were used here to represent for mammalian cells. The glucose concentration was set at 10 g/L and the collagen concentration was fixed at 2.25 g/L. The effects of entrapped cells on the effective diffusivities of collagen gels were listed in Figure 3. The statistical data were expressed as mean±SD. In cell-free collagen gel, this coefficient was determined as 1.441±0.017×10-6 cm2/s, which is much lower than the corresponding molecular diffusivity (6.7×10-6 cm2/s). At a low cell density of 0.5×106 cells/mL, the effective diffusion coefficient was 1.512± 0.042×10-6 cm2/s for collagen gels entrapped with dead cells, which was an equivalent of that for cell-free collagen gel. In contrast, this value decreased to 0.502±0.002×10-6 cm2/s in the comparatively contracted gel after 24 h interaction of cells with collagen gels. While at a high cell density of 5.0×106 cells/mL, this coefficient was 0.799±0.013×10-6 cm2/s for non-contraction gel and only 0.231±0.017×10-6 cm2/s for the contracted gel. It seems that collagen contraction resulted in a significant decrease of the effective diffusivity and should be the main reason for reduced diffusion across gels other than the obstruction of cell masses.
By measuring the effective diffusivities, we know the diffusion situation inside collagen gels with or without the interaction of cells. And it is common knowledge that the diffusion across membranes was mainly determined by the transmembrane concentration gradient and the molecular sizes of both solutes and membrane pores. Thus, the integrated diffusion across both membrane and collagen gel can be reflected by cell cultivation itself within such bioreactor. Cell viability was first checked by cultivation of cells in hollow fibers with variant membrane pore size and different cell concentration. Cell function was later investigated.
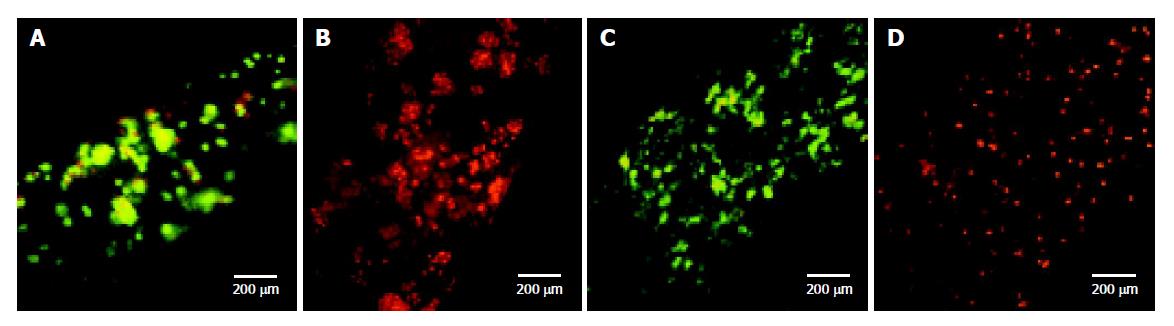
To feature the cell surviving abilities in such configured system, both rat hepatocytes and KB cells were employed here. Hepatocyte cell culture at two different cell densities was employed in hollow fibers with three featured pore sizes, 0.1 µm, MWCO of 30 and 100 ku, respectively. Initial harvest viability of hepatocyte was about 85%. At the culture time of 78 h, the cell matrices were carefully taken out from the hollow fibers for FDA/EB staining. Hepatocytes at an inoculated cell density of 1×106 cells/mL almost maintain the initial viability by cultivated in hollow fibers with MWCO of 100 ku (Figure 4A) and in microporous hollow fibers with pore size of 0.1 µm (pictures are not shown here), but all appeared dead in the hollow fibers with MWCO of 30 ku (Figure 4B). No hepatocytes were survived when entrapped into the hollow fibers for 78 h at a higher cell density of 5×106 cells/mL, as expected from our early observation in gel contraction at this cell density. Considering their multiplication, KB cells was inoculated at a low cell density of 0.4×106 cells/mL in hollow fibers with MWCO of either 100 or 30 ku here. The viability of KB cells was rather high in hollow fibers with MWCO of 100 ku (Figure 4C) and it can also be noticed that KB cells propagate well according to the more concentrated cell density than the initial one (the similar density as that in Figure 4D). In contrast to the primary hepatocytes, KB cells are more robust to survive and can propagate quickly even with a basic mammalian cell culture medium, so its viability is usually above 98% on the condition without critical environmental problems such as nutrition shortage and physiological chaos. Even so, KB cells did not survive in hollow fibers with MWCO of 30 ku (Figure 4D) and its cell density was almost the same as the initial one. Viability measurement showed that cells grew well in hollow fibers with MWCO of 100 ku which provides a better diffusion property than hollow fibers with MWCO of 30 ku.
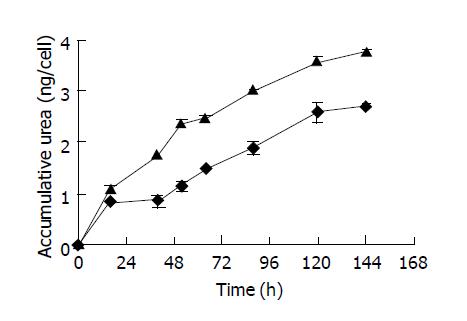
As the hepatocyte viabilities were similar in hollow fibers with either MWCO of 100 ku or pore size of 0.1 µm at the cell density of 1×106 cells/mL, cell functions were examined to reflect the diffusion ability in such system. Culture media were changed between 48 and 72 h and media samples were taken every 12 h. The urea production was used a marker for the liver-specific function of hepatocytes. The accumulative urea production throughout the culture period was calculated and shown in Figure 5. It can be seen that hepatocytes produced more urea in the microporous hollow fibers than in the ultrafiltration hollow fibers. By performing linear fit on data points, average urea production were determined and summarized in Table 1. Hepatocytes entrapped inside the microporous hollow fibers exhibited higher urea production (31.2 pg/cell per h) and one-third lower of urea production inside the ultrafiltration hollow fibers.
| Hollow fiber feature | Microporous hollow fiber | Ultrafiltration hollow fiber |
| Average urea production (pg/cell per h) | 31.2 | 20.7 |
This paper discussed the possible diffusion barrier in the mammalian cell culture system featured by entrapment of cells into cell matrix in hollow fibers. We focused on two major mass transfers by diffusion in static culture. One was the diffusion across cell-matrix gel and the other was the diffusion across membranes. Because diffusion across the membranes has been well studied, the possible diffusion barrier caused by cell mediation was of great consideration in this paper. Also the integrated diffusion across both collagen gel and membranes was also evaluated through cell culture.
The cell-mediated collagen gel contraction and its effect on glucose diffusivities were first determined. Two types of mammalian cells, primary hepatocytes and continuous KB cells, were mixed with collagen gel and then incubated for a couple days to detect gel contraction. It was found that collagen contraction was caused not only by mediation of primary cells, as previously reported, but also by mediation of continuous cells, although the former was more significant. Such gel contraction was caused by the interaction between mammalian cells and cell matrix and could also serve as a crude estimate of cell viability. The measurement of the effective diffusion coefficients across cell-matrix gels reflected that the solute mobility by diffusion was inhibited more significantly by gel contraction than by the obstruction of entrapped cell mass. This is most possibly attributed to the densification of collagen gel. Thereby, it can be reached that the presence of cell masses together with cell-mediated gel contractions caused the remarkable diffusion resistance but the gel contraction was the major factor.
Cell viability measurement was then applied for evaluating diffusions in static culture of primary hepatocyte and continuous KB cells by collagen entrapment within hollow fibers. Hepatocytes maintained almost the initial viability in either microporous hollow fibers or ultrafiltration hollow fibers with higher MWCO of 100 ku but were all dead in ultrafiltration hollow fibers with lower MWCO of 30 ku. Similarly, KB cells presented quite high cell viability and even a well proliferation in hollow fibers with MWCO of 100 ku but did not survive in hollow fibers with MWCO of 30 ku. Based on the well studied mass transfer across membranes, the cell death in hollow fibers with lower MWCO is due to the exclusion of solutes with large molecular weight, for example, BSA with molecular weight of 67 ku. The shortage of such nutrients most possibly causes cell death.
Noticing that KB cells present much higher viability even at a high cell density due to propagation while hepatocytes cannot survive at the density of only 5×106 cells/mL, this could be contributed to the more roughly requirement of KB cells than primary hepatocyte, for example, primary cells usually need more nutrients for maintaining in vitro such as proteins or hormones. As the glucose effective diffusivity across gels decreases with cell density, it is understandable that hepatocyte viability also decreases with the inoculated cell density, or in another word, the limited mass transport by diffusion is prevalent inside collagen gels entrapped with high cell density of viable cells. Actually, in Nyberg’s paper[30], around 50% of reduction on hepatocyte viability within one day culture was reported without further investigation where hepatocytes were statically cultivated in collagen noodles at a cell density of 4.4×106 cells/mL. This decrease in cell viability was quite unexpected in comparison with the high viability in his hollow fiber bioreactor running[31], indicating the possible limited diffusion of nutrients across gels in static culture. And it is worth mentioning that our lower viability in our hollow fiber culture at cell density of 5×106 cells/mL in comparison to their hepatocyte viability of 45%[30] could due to the extra membrane barrier and different medium composition and no daily medium changing.
As hepatocytes exhibit similar viabilities in hollow fibers featured respectively by large MWCO and microporous, liver-specific function on urea secretion was further examined to compare the effect of diffusion. The urea production was much higher in microporous hollow fibers than in the ultrafiltration hollow fibers with MWCO of 100 ku. This corresponded well with our previous results that higher liver-specific function was performed in bioartificial livers with microporous hollow membranes. Thus, by this experiment, it could be concluded that higher liver-specific functions were closely related to better diffusion. We did not discuss the possible diffusion resistance caused by a boundary layer or a stagnant film on surface of membranes or collagen gels, however, by considering the same materials (polysulfone and collagen gel) and the same configurations among all these hollow fiber systems, it is reasonable to exclude the effect of the diffusion in such boundary layer.
In summary, both cell-matrix gel and membrane compartment contribute to diffusion resistance in static culture of mammalian cells by gel entrapment within hollow fibers. Cell culture of continuous KB cell and primary hepatocyte confirm the cell-mediated gel contraction and the limited transportation of nutrients by diffusion.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Jauregui HO, Chowdhury NR, Chowdhury JR. Use of mammalian liver cells for artificial liver support. Cell Transplant. 1996;5:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Gloeckner H, Lemke HD. New miniaturized hollow-fiber bioreactor for in vivo like cell culture, cell expansion, and production of cell-derived products. Biotechnol Prog. 2001;17:828-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Luque S, Mallubhotla H, Gehlert G, Kuriyel R, Dzengeleski S, Pearl S, Belfort G. A new coiled hollow-fiber module design for enhanced microfiltration performance in biotechnology. Biotechnol Bioeng. 1999;65:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Wolf CF, Lauffer LL. Design and fabrication of a capillary cell culture chamber for the study of convective flow. Int J Artif Organs. 1986;9:25-32. [PubMed] |
| 6. | Piret JM, Cooney CL. Immobilized mammalian cell cultivation in hollow fiber bioreactors. Biotechnol Adv. 1990;8:763-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nyberg SL, Shatford RA, Peshwa MV, White JG, Cerra FB, Hu WS. Evaluation of a hepatocyte-entrapment hollow fiber bioreactor: a potential bioartificial liver. Biotechnol Bioeng. 1993;41:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Sielaff TD, Hu MY, Amiot B, Rollins MD, Rao S, McGuire B, Bloomer JR, Hu WS, Cerra FB. Gel-entrapment bioartificial liver therapy in galactosamine hepatitis. J Surg Res. 1995;59:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Jauregui HO, Muller TE. Long-term cultures of adult mammalian hepatocytes in hollow fibers as the cellular component of extracorporeal (hybrid) liver assist devices. Artif Organs. 1992;16:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Stanness KA, Guatteo E, Janigro D. A dynamic model of the blood-brain barrier "in vitro". Neurotoxicology. 1996;17:481-496. [PubMed] |
| 11. | Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 225] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Ala-Uotila S, Marjamäki A, Matikainen MT, Jalkanen M. Use of a hollow fiber bioreactor for large-scale production of alpha 2-adrenoceptors in mammalian cells. J Biotechnol. 1994;37:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Sielaff TD, Nyberg SL, Rollins MD, Hu MY, Amiot B, Lee A, Wu FJ, Hu WS, Cerra FB. Characterization of the three-compartment gel-entrapment porcine hepatocyte bioartificial liver. Cell Biol Toxicol. 1997;13:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Bridge MJ, Broadhead KW, Hitchcock RW, Webb K, Tresco PA. A simple instrument to characterize convective and diffusive transport of single hollow fibers of short length. J Membr Sci. 2001;183:223-233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Nagy E, Hadik P. Analysis of mass transfer in hollow-fiber membranes. Desalination. 2002;145:147-152. [DOI] [Full Text] |
| 16. | Wickramasinghe SR, Garcia JD, Han B. Mass and momentum transfer in hollow fibre blood oxygenators. J Membr Sci. 2002;208:247-256. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Dionne KE, Cain BM, Li RH, Bell WJ, Doherty EJ, Rein DH, Lysaght MJ, Gentile FT. Transport characterization of membranes for immunoisolation. Biomaterials. 1996;17:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Boyd RF, López M, Stephens CL, Vélez GM, Ramírez CA, Zydney AL. Solute washout experiments for characterizing mass transport in hollow fiber immunoisolation membranes. Ann Biomed Eng. 1998;26:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Catapano G. Mass transfer limitations to the performance of membrane bioartificial liver support devices. Int J Artif Organs. 1996;19:18-35. [PubMed] |
| 20. | Meng Q, Zhang G, Wu D. Hepatocyte culture in bioartificial livers with different membrane characteristics. Biotechnol Lett. 2004;26:1407-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Calabr V, Curcio S, Iorio G. A theoretical analysis of transport phenomena in a hollow fiber membrane bioreactor with immobilized biocatalyst. J Membr Sci. 2002;206:217-241. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Seglen PO. Methods in cell biology. New York: Academic Press 1976; 30-83. |
| 23. | Friend JR, Wu FJ, Hansen LK, Remmel RP, Hu WS. Tissue engineering methods and protocols in: Moran JR, Yarmush ML, eds. Methods in molecular medicine. Totowa: Humana Press Inc 1998; 245-252. |
| 24. | Meng Q. Hypothermic preservation of hepatocytes. Biotechnol Prog. 2003;19:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Hannoun BJ, Stephanopoulos G. Diffusion coefficients of glucose and ethanol in cell-free and cell-occupied calcium alginate membranes. Biotechnol Bioeng. 1986;28:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Yu S, Olsen CE, Marcussen J. Methods for the assay of 1,5-anhydro-D-fructose and α-1,4-glucan lyase. Carbohydr Res. 1998;305:73-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Teixeira JA, Mota M, Venancio A. Model identification and diffusion coefficients determination of glucose and malic acid in calcium alginate membranes. Chem Eng J. 1994;56:B9-B14. |
| 28. | Taveira P, Mendes A, Costa C. On the determination of diffusivity and sorption coefficients using different time-lag models. J Membr Sci. 2003;221:123-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Rutherford SW, Do DD. Review of Time Lag Permeation Technique as a Method for Characterisation of Porous Media and Membranes. Adsorption. 1997;3:283-312. [RCA] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Nyberg SL, Shatford RA, Payne WD, Hu WS, Cerra FB. Primary culture of rat hepatocytes entrapped in cylindrical collagen gels: an in vitro system with application to the bioartificial liver. Rat hepatocytes cultured in cylindrical collagen gels. Cytotechnology. 1992;10:205-215. [PubMed] |
| 31. | Shatford RA, Nyberg SL, Meier SJ, White JG, Payne WD, Hu WS, Cerra FB. Hepatocyte function in a hollow fiber bioreactor: a potential bioartificial liver. J Surg Res. 1992;53:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |