Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1554
Revised: August 14, 2004
Accepted: October 5, 2004
Published online: March 14, 2005
AIM: To reduce the possibility of gastroduodenal complications. The purpose of this retrospective study was to survey the literature and compare and discuss the incidence of post-transarterial embolization (TAE) gastroduodenal complications.
METHODS: We found reports describing 280 cases of hepatocellular carcinoma with TAE procedures done during the past 4 years and selected all of them for our study. Amongst these cases, 86 were suspected of suffering gastroduodenal complications within one month of post-TAE treatment. Fifteen of these cases were proved by pan-endoscopy to have gastroduodenal erosions or ulcerations. We reviewed the angiographic pictures in patient records to evaluate the possibility that anatomic and technical skill factors could explain the complications.
RESULTS: Amongst the 15 cases, 9 were primary lesions of the antrum and prepylorus; 4 had duodenal ulcer or erosions; 2 had mid-body lesions; none showed a lesion at the fundus or cardia region. Three cases had not received TAEs using our ideal method, and may be associated with possible regurgitation of gel-foam pieces into the right or left gastric arteries. Two cases involved sub-selective embolization at a distal point on the hepatic artery; one case was found by angiography to have complete occlusion of the celiac trunk.
CONCLUSION: Comparing our results with past cases of post-TAE gastroduodenal complications, we surmise that our relatively low incidence (5.3%) of gastric complications might be explained by our concerted efforts to improve our technical skills in multi-sequential, selective and super-selective approaches to the embolization of tumor vessels.
- Citation: Leung TK, Lee CM, Chen HC. Anatomic and technical skill factor of gastroduodenal complication in post-transarterial embolization for hepatocellular carcinoma: A retrospective study of 280 cases. World J Gastroenterol 2005; 11(10): 1554-1557
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1554
Transcatheter embolization for hepatocellular tumors has been performed worldwide for over 20 years. A Japanese study[1,2] suggested that post-transarterial embolization (TAE) complications are mainly due to reflux of gel-foam pieces into the right gastric artery and to special anatomic factors (e.g., the right gastric artery is located too distal from the proper hepatic artery). In our 4-year study, we evaluated cases that had been reported in the literature and which resulted in post-TAE-induced gastroduodenal complications, as seen on endoscopy. In the current study we compare and discuss these cases.
From January of 2000 to December of 2003, hundreds of cases in our hospital were diagnosed as hepatocellular carcinoma by fine needle biopsy, characteristic elevation of alpha-fetoprotein, or characteristic imaging findings (including three phases CT scans and diagnostic angiography). These patients received our TAE or transarterial chemotherapeutic embolization (TACE). In order to prevent the possible influence of adverse gastroduodenal effects due to anticancer drugs[3], we excluded cases of combined treatment with chemotherapeutic, anticancer drugs (TACE). A total of 280 TAE cases were evaluated in this retrospective study.
Our TAE procedures involved administration of a mixture of lipiodol and 2 mg of gentamicin in a 20 cc syringe and a mixture of gel-foam pieces with contrast medium in another 20 cc syringe.
In order to prevent gastroduodenal complications, to preserve hepatic function and to decrease other possible adverse effects, most procedures involved placement of a 4- to 5-fr. catheter or a microcatheter, superselectively or selectively, so that embolization was as close as possible to the tumor vessels. The mixtures were transferred into smaller 2 cc syringes to achieve a slow and steady controlled injection. The procedures were closely monitored under fluoroscopy in order to minimize backflow of lipiodol and gel-foam powder into the right gastric, left gastric or gastroduodenal arteries.
As part of our retrospective study, we recorded cases where patients had suffered gastric discomfort after the TAE procedure. There were 86 cases that received pan-endoscopy of the upper gastrointestinal tract (from esophagus to duodenum). Fifteen cases were chosen with positive findings of acute gastroduodenal erosions and ulcerations and which met the following criteria: (1) no history of peptic ulcer in the past 3 years; (2) patients began to have epigastric pain, nausea, black color stool or other gastric symptoms less than one month after TAE.
Amongst the 15 cases, 11 (73%) were suffering from GI symptoms and received pan-endoscopy with positive findings less than one week after TAE; 4 (27%) received pan-endoscopy more than one week but less than one month after TAE.
Endoscopic findings for the above study group included active stages of erosion and ulceration. None were found to involve active bleeding. The location of the main gastroduodenal lesions[3-5] we found, is listed in Table 1.
| Location | Case number |
| Fundus and cardia | 0 |
| Mid-body | 2 |
| Antrum and pre-pylorus | 9 |
| Duodenal ulcer or erosions | 4 |
| P | 0.77 |
Under arterial phase imaging, different patterns of anatomy of the proper hepatic artery, right hepatic artery and right gastric artery were found[6]:
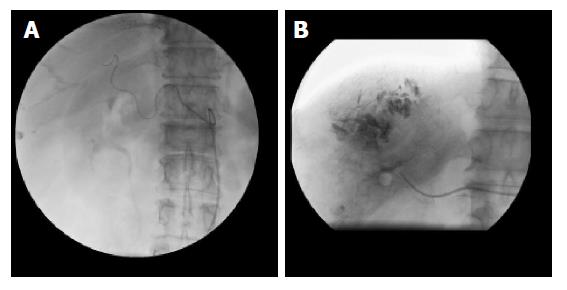
(1) Ten of the fifteen cases (66.7%) showed the most common anatomic form of three branches arising from the celiac trunk. The right gastric artery arose from the proper hepatic artery. The left hepatic artery divided from the proper hepatic artery. Amongst these patients, there were 4 cases with combined left and right lobe hepatic tumors. Each TAE procedure was done using the super-selective or selective principle (Figure 1A and B) with slow steady injection under fluoroscopic monitoring. Placement of the catheter was into the branches or the main trunk of the right hepatic artery (8 cases) or the left hepatic artery (4 cases). The observation reports are as follows: (1) two cases were completed using a non-ideal method, the sub-selective principle, where injection was made into the distal portion of the proper hepatic artery. Post-TAE angiographic injections of the above cases showed no diminished blood flow of the original, visible, right and left gastric arteries; (2) Two cases (13.3%) showed complete displacement of the origin of the right hepatic artery to the superior mesenteic artery. The left hepatic and left gastric artery divided from the common hepatic artery. The right gastric artery arose from the left hepatic artery. TAE procedures were completed with selective injection (slow steady injection under fluoroscopic monitoring) into the right hepatic artery. Post-TAE angiographic injections showed no diminished blood flow in the right and left gastric arteries; (3) Two cases (13.3%) showed a three-branched celiac trunk with the right gastric artery arising from the left hepatic artery. The right hepatic artery divided from the proper hepatic artery. TAE procedures were completed with selective or super-selective injections into the right hepatic artery, again with slow steady injection under fluoroscopic monitoring. Post-TAE angiographic injections showed no diminished blood flow in the right and left gastric arteries; (4) there was one case (6.7%) with complete occlusion of the celiac trunk. Arterial angiography showed the absence of the common hepatic artery and its branches. The catheter was inserted so as to approach the right lobe of the hepatic tumor via a collateral branch of the SMA. The TAE procedure was completed without angiographic mapping of the right and left gastric arteries.
Three patients did not receive TAE under ideal standard conditions. Of these three, 2 were in group 1 and 1 in group 4. All of them were associated with higher risk for gastroduodenal complications because of the imperfect technique that was used. A χ2, comparing these 3 to the other 12 cases, which had involved the use of the selective or super-selective TAE techniques, indicated that there was no statistically significant difference in the outcomes (P = 0.16; Table 2).
| Technique | Non-superselective/nonselective TAE | Super-selective-or selective TAE | P |
| Case number | 3 | 12 | 0.16 (>0.05) |
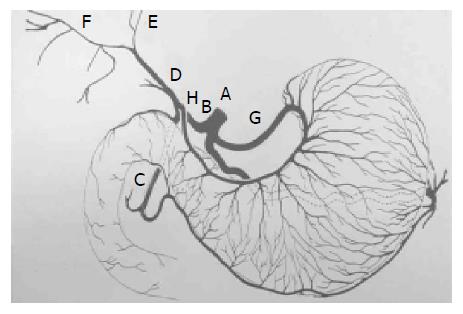
Usually, celiac trunk (A) gives rise to the common hepatic artery (B) and continue with proper hepatic artery (D), following the origin of the gastroduodenal artery (C). This artery is usually short and divides into the right (F), left (E) and, occasionally middle hepatic arteries. In others, one or more of the hepatic artery branches have a partially or totally displaced origin. The proper hepatic artery also frequently gives rise to the right gastric artery (H). Rarely, the origin of the entire hepatic blood supply is displaced to the superior mesenteric artery.
The right gastric artery is not always visible at angiography unless it is providing a collateral blood supply to the left gastric artery[6]. It is a small vessel that arises from the proper hepatic artery shortly after the origin of the gastroduodenal artery (C). It may also arise from the proximal left hepatic artery, especially when the right hepatic circulation is displaced to the superior mesenteric artery. The right gastric arteries are mostly anastomoses with the posterior branches of the left gastric artery (G). They mainly supply the pylorus and distal posterior surface of the stomach.
Tsuchigame and Takahashi[1] suggested that post-TAE gastric lesions are basically due to the backflow of the embolic materials into the gastric artery and the consequent decrease in gastric mucosal blood flow may complicate gastric erosion or ulceration. According to their study, the incidence of post-TAE gastric complication is about 9%.
Nakamura and Hashimoto also discussed post-TAE gastric complications[8]. They concluded that some anatomic variations - such as, the right gastric artery branches distally from the proper artery or its branch, or the accessory left gastric artery arises from the left hepatic artery - are the ones that are most likely to be associated with a high incidence of post-TAE gastric lesion.
Amongst the 15 cases of post-TAE gastroduodenal lesions, none showed complications in the gastric fundus and cardia region, suggesting a lesser association of complications with the left gastric artery. A high incidence of lesions was found at the antrum and prepylorus, which may reflect a greater association with the right gastric artery embolization[9,10].
However, these differences in the incidence of post-TAE complication and the gastroduodenal locations were not significant (Table 1).
According to our retrospective study of angiographic findings, only the two cases involving subselective proper hepatic artery embolization in Group 1 appear to complicate non-observable gel-foam[11] reflux into the right gastric artery. On the other hand, the post-TAE consequence of gastroduodenal complications in Group 4 is unavoidable due to the unusual anatomic situation.
Angiographic reviews of Groups 2 and 3 appeared to involve the technical skills and safety factors during the embolization procedures and were proved by post-TAE angiographic views of the normal right gastric artery.
From January of 2000 to December of 2003, we improved our TAE procedure by incorporating segmental or sub-segmental embolizations. We strictly obeyed the principle of selective or superselective procedures during the embolizations. We achieved a rather low incidence (5.3%) of post-TAE gastroduodenal complications, which compared favorably to the 9% rate we found for published reports. In these reports, 3 of 280 cases (-1%) could be explained by non-ideal technical skills and anatomic factors, but 12 cases (4.3%) could not be explained.
Our findings indicate that there is no significant correlation between gastroduodenal complications[12] and our imperfect technical embolization procedures (Table 2).
However, there still remain gastroduodenal complications[13] (4.3%), beyond those attributable to anatomic factors[14] and technical skills[6].
Stress ulcer is one other possible etiology. There are at least five types of stress ulcers[15] causing disturbances in the stomach and intestines. The pathogenesis of stress-induced lesions is probably multifactorial. We deduce that post-TAE pain sensation may be one stress-inducing factor. During stressful events[16], normal protective mechanisms are altered, including epithelial turnover in the gastric mucosa and secretion of mucus and bicarbonate. These events, combined with the release of various mediators (e.g., arachidonic acid metabolites, cytokines, oxygen-free radicals), result in erosions that may progress to ulceration and bleeding[15,16]. Reductions in mucosal blood flow may also be important in ulcer formation. Alterations in blood flow at the microcirculatory level may initiate changes that result in erosions that can progress to ulceration and bleeding.
Edited by Guo SY Language Editor Elsevier HK
| 1. | Tsuchigame T, Takahashi M, Watanabe O, Yoshimatsu S, Yamashita Y, Uozumi H, Ueno S, Hirota Y. Pathogenesis and prevention of gastrointestinal complications following transcatheter arterial embolization. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50:798-803. [PubMed] |
| 2. | Ishigaki H, Suto T, Sasaki D, Tsushima K, Higuchi S, Baba T, Sano M, Munakata A, Yoshida Y, Takagi S. Factors of gastric lesions following after transcatheter arterial embolization for primary hepatoma. Nihon Shokakibyo Gakkai Zasshi. 1990;87:57-61. [PubMed] |
| 3. | Matsuura T, Tsukamoto Y, Nakata H, Nakayama T, Shirakawa F, Okazaki I, Suzuki H. Complication of therapeutic transcatheter hepatic artery embolization for hepatoma--case of developing an extensive acute gastric ulcer. Gan No Rinsho. 1983;29:A-11, 164-167. [PubMed] |
| 4. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Hirakawa M, Iida M, Aoyagi K, Matsui T, Akagi K, Fujishima M. Gastroduodenal lesions after transcatheter arterial chemo-embolization in patients with hepatocellular carcinoma. Am J Gastroenterol. 1988;83:837-840. [PubMed] |
| 6. | Lee KH, Sung KB, Lee DY, Park SJ, Kim KW, Yu JS. Transcatheter arterial chemoembolization for hepatocellular carcinoma: anatomic and hemodynamic considerations in the hepatic artery and portal vein. Radiographics. 2002;22:1077-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Stewart R. Reuter, M.D. Gastrointestinal Radiology 1986: 1 (Saunders monographs in clinical radiology). . |
| 8. | Nakamura H, Hashimoto T, Oi H, Sawada S, Furui S. Prevention of gastric complications in hepatic arterial chemoembolization. Balloon catheter occlusion technique. Acta Radiol. 1991;32:81-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Bradley EL, Goldman ML. Gastric infarction after therapeutic embolization. Surgery. 1976;79:421-424. [PubMed] |
| 10. | Wells JJ, Nostrant TT, Wilson JA, Gyves JW. Gastroduodenal ulcerations in patients receiving selective hepatic artery infusion chemotherapy. Am J Gastroenterol. 1985;80:425-429. [PubMed] |
| 11. | Tamura S, Kihara Y, Yuki Y, Sugimura H, Shimizu T, Nishikawa T, Asato M, Adjei ON, Tong XQ, Watanabe K, Hasui Y. Lipiodolized gelatin sponge: a simple method to make gelatin sponge radiopaque. Radiat Med. 1998;16:13-15. [PubMed] |
| 12. | Hall DA, Clouse ME, Gramm HF. Gastroduodenal ulceration after hepatic arterial infusion chemotherapy. AJR Am J Roentgenol. 1981;136:1216-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Tsuchigame T, Takahashi M, Bussaka H, Miyawaki M, Fukui K, Yasunaga T, Nanakawa S, Miyao M. Acute gastric lesions following transcatheter arterial embolization of hepatic malignancies. Nihon Igaku Hoshasen Gakkai Zasshi. 1984;44:1501-1507. [PubMed] |
| 14. | Charnsangavej C, Carrasco CH, Wallace S, Richli W, Haynie TP. Hepatic arterial flow distribution with hepatic neoplasms: significance in infusion chemotherapy. Radiology. 1987;165:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Wightkin WT. Stress ulcer: pathophysiology and prevention. Am J Hosp Pharm. 1980;37:1651-1655. [PubMed] |
| 16. | Liu GS, Huang YX, Li SW, Pan BR, Wang X, Sun DY, Wang QL. Experimental study on mechanism and protection of stress ulcer produced by explosive noise. World J Gastroenterol. 1998;4:519-523. [PubMed] |