Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1535
Revised: August 26, 2004
Accepted: December 1, 2004
Published online: March 14, 2005
AIM: To examine the etiology and pathophysiology in human ischemic colitis from the viewpoint of ischemic factors such as hypoxia-inducible factor 1 alpha (HIF-1 alpha and vascular endothelial growth factor (VEGF).
METHODS: Thirteen patients with ischemic colitis and 21 normal controls underwent colonoscopy. The follow-up colonoscopy was performed in 8 patients at 7 to 10 d after the occurrence of ischemic colitis. Biopsy samples were subjected to real-time RT-PCR and immunohistochemistry to detect the expression of HIF-1 alpha and VEGF.
RESULTS: HIF-1 alpha and VEGF expression were found in the normal colon tissues by RT-PCR and immunohistochemistry. HIF-1 alpha and VEGF were overexpressed in the lesions of ischemic colitis. Overexpressed HIF-1 alpha and VEGF RNA quickly decreased to the normal level in the scar regions at 7 to 10 d after the occurrence of ischemic colitis.
CONCLUSION: Constant expression of HIF-1 alpha and VEGF in normal human colon tissue suggested that HIF-1 alpha and VEGF play an important role in maintaining tissue integrity. We confirmed the ischemic crisis in ischemic colitis at the molecular level, demonstrating overexpression of HIF-1 alpha and VEGF in ischemic lesions. These ischemic factors may play an important role in the pathophysiology of ischemic colitis.
- Citation: Okuda T, Azuma T, Ohtani M, Masaki R, Ito Y, Yamazaki Y, Ito S, Kuriyama M. Hypoxia-inducible factor 1 alpha and vascular endothelial growth factor overexpression in ischemic colitis. World J Gastroenterol 2005; 11(10): 1535-1539
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1535.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1535
Ischemic colitis is a vascular condition of inadequate blood flow in the colon, which leads to colonic inflammation and can produce significant morbidity and mortality. Two well- known risks -factors of ischemic colitis are vascular factors and bowel factors[1,2]. The vascular factors are related to systemic arteriosclerosis based on hypertension, diabetes mellitus, hyperlipidemia and thrombus, embolus, and vasculitis. It is also associated with hypovolemia, hypotension and the use of vasoconstricting drugs[1,3]. The bowel factors are colon spasm, constipation, fecal impaction and prior history of abdominal surgery[1,2]. The well-known pathological findings are degeneration, deciduation, necrosis, regeneration of the epithelial layer, congestion, fibrin thrombus in blood capillaries, leukocyte invasion in the lamina propria and bleeding, edema, and exudates in the submucosal layer[4]. Ischemic crisis is believed to occur in the tissue in ischemic colitis. However, there is no precise pathophysiological examination for so called “ischemic crisis” in this disease.
Drastic adaptation reactions are quickly induced to maintain homeostasis and preserve life, when the tissues are in an ischemic condition. Recently, several important discoveries have been made; the detection of hypoxia inducible factor 1 alpha (HIF-1 alpha and vascular endothelial growth factor (VEGF)[5,6]. Increased concentrations of intracellular HIF-1 alpha molecules occur after hypoxia as a result of reduced degradation by the ubiquitin proteasome pathway[7,8]. Binding HIF-1 alpha to the hypoxia response element of several “HIF-regulated genes” results in the increased transcription of several proteins involved in angiogenesis, glycolysis, erythropoiesis, the inhibition of apoptosis, and monocyte-related inflammation[9,10]. VEGF is one of the most important factors for angiogenesis. VEGF is produced by the promoter signals from the HIF-1 alpha complex, secreted as a paracrine factor to the extracellular space, and binds to specific receptors, KDR/Flk-1[11-13]. The signal transduction after self-phosphorylation of the VEGF receptor leads to angiogenesis due to endothelial cell proliferation[14].
The aim of the present study was to examine the etiology and pathophysiology in human ischemic colitis from the viewpoint of these ischemic factors.
Thirteen patients with ischemic colitis diagnosed by endoscopy (3 males and 10 females, mean age 63.2) participated in the present study. The ischemic lesion was located from the descending to the sigmoid colon in twelve cases and from the ascending to the transverse colon in one case. A total of three biopsy specimens (two from the lesion and one from normal rectal mucosa) were taken from each patient. One tissue specimen from the lesion and one from the normal rectal mucosa were put into a 1.5 mL Microfuge tube with 100 uL of RNA stabilizer agent (RNAlater, Qiagen, Valencia, CA, USA), stored at -80 °C, and subjected to real-time RT-PCR analysis. One specimen from the lesion was fixed in 10% buffered formalin (pH 7.2). Ordinary paraffin sections were cut, stained with hematoxylin and eosin and subjected to histological analysis. The follow-up colonoscopy was performed in 8 patients at 7-10 d after the first endoscopy. In these cases, two biopsy specimens (one for real-time RT-PCR and one for histological analysis) were taken from the cured lesion. Twenty-one normal controls (14 males and 7 females, mean age 67.1) were also chosen from subjects, who received total colonoscopy for colon cancer screening, performed by the Second Department of Internal Medicine, Faculty of Medical Sciences, University of Fukui or Department of Internal Medicine, and Tannan Regional Medical Center. All normal controls had normal findings in total colonoscopy. A total of eight biopsy specimens (two from the terminal ileum, two from the ascending colon, two from the descending colon and two from the rectum) were taken from normal controls. One specimen from each region was subjected to real-time RT-PCR and one to histology. This work was performed according to the principles of the Declaration of Helsinki and consent was obtained from each individual after a full description of the nature and protocol of the study.
The tissue specimens were homogenized and an RNA extracted using RNA extraction kit (RNeasy Mini Kit, Qiagen, Valencia, CA, USA), according to the manufacturer’s protocol. HIF-1 alpha and VEGF expression levels were assessed by real-time quantitative RT-PCR methods with the ABI prism 7700 system (TaqMan PCR, Applied Biosystems, Foster city, CA, USA) using Assays-on-Demand primers and probe sets Hs00153153m1 and Pre-developed TaqMan Assay reagent 4339277F respectively. The thermal cycling conditions included 48 °C for 30 min and 95 °C for 10 min, followed by 50 cycles of amplification at 95 °C for 15 s and 60 °C for one minute. The PCR products were also examined by 2% agarose gel electrophoresis. The gels were stained with ethidium bromide to confirm successful amplification of the expected sequences. Human glyceraldehydes-3-phosphate dehydrogenase (GAPDH) RNA was measured as a control using Pre-developed TaqMan Assay reagent 4310884E (Applied Biosystems, Foster City, CA, USA). RNA for GADPH was used as an endogenous control. The amount of HIF-1 alpha or VEGF RNA was normalized to the level of GADPH by the ratio of HIF-1 alpha or VEGF to GAPDH.
HIF-1 alpha and VEGF expression were analyzed using a DAKO Envision plus kit (Dako Cytomation, Carpinteria, CA, USA). The primary antibodies used in this study were sc-10790 (rabbit, 1:50 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for HIF-1 alpha and sc-152 (rabbit, 1:25 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) for VEGF. Sections (4 um thick) were dewaxed and endogenous peroxidase activity was quenched with 3% H2O2 for 30 min. Antigen retrieval was achieved by heat treatment at 70 °C for 3 h. Sections were incubated with a blocking agent (Protein Block Serum-free: ×0909, Dako Cytomation, Carpinteria, CA, USA) for 20 min to reduce non-specific reactions. After washing with tris buffered saline (TBS), the primary antibodies were applied for 2 h and washed in TBS. Sections were incubated with a secondary goat anti-rabbit antibody for 60 min and washed in TBS. The color was developed by 7 min incubation with DAB solution and the sections were weakly counterstained with hematoxylin. Normal rabbit immunoglobulin G was substituted for the primary antibody as the negative control at the same concentration. As positive controls, we used advanced gastric or colon cancer cases with intense cancer cells of HIF-1 alpha and VEGF expression.
Statistical analysis was performed using the Statistical Package for the Biosciences (SPBS) version 9.44 software (Murata, Kyoto, Japan). Differences among groups were assessed using the non-parametric Mann-Whitney U test and the Kruskal-Wallis rank-sum test. Significance was set at P<0.05.
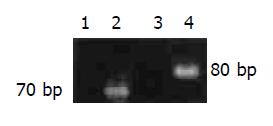
HIF-1 alpha and VEGF PCR products were found in the normal colon tissues (Figure 1) and the relative amounts of expression in each region of the colon were shown in Table 1. There were no significant differences in the expression levels among the regions.
| Terminal ileum | Ascending colon | Descending colon | Rectum | P1 | |
| HIF-1 alpha/GAPDH | 1.18±0.45 | 1.01±0.38 | 1.12±0.49 | 1.40±0.71 | 0.180 (NS) |
| VEGF/GAPDH | 2.49±2.61 | 1.32±0.98 | 1.54±1.26 | 1.55±1.28 | 0.479 (NS) |
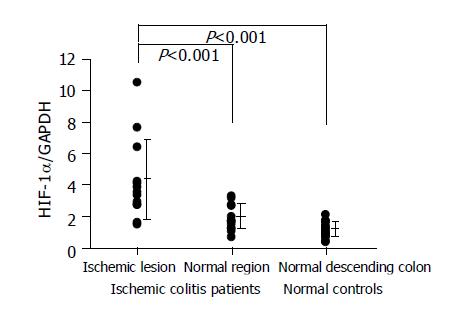
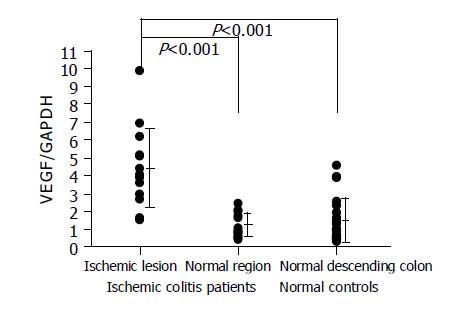
HIF-1 alpha was overexpressed in the lesions of ischemic colitis patients. The relative amounts of HIF-1 alpha RNA levels were significantly higher in the ischemic lesions than in the normal region in the same patient or in the normal descending colon tissues of the normal controls (Figure 2). VEGF was also overexpressed in the lesions of ischemic colitis patients. The relative amounts of VEGF RNA levels were significantly higher in the ischemic lesions than in a normal region in the same patient or in the normal descending colon tissues of the normal controls (Figure 3).
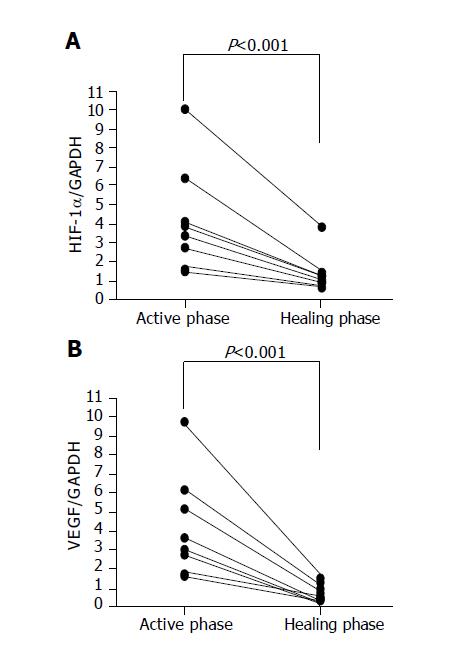
The colonoscopic findings returned to almost the normal state with scars at 7 to 10 d after the occurrence of ischemic colitis. Overexpressed HIF-1 alpha and VEGF RNA quickly decreased to normal levels in the scar regions (Figure 4).
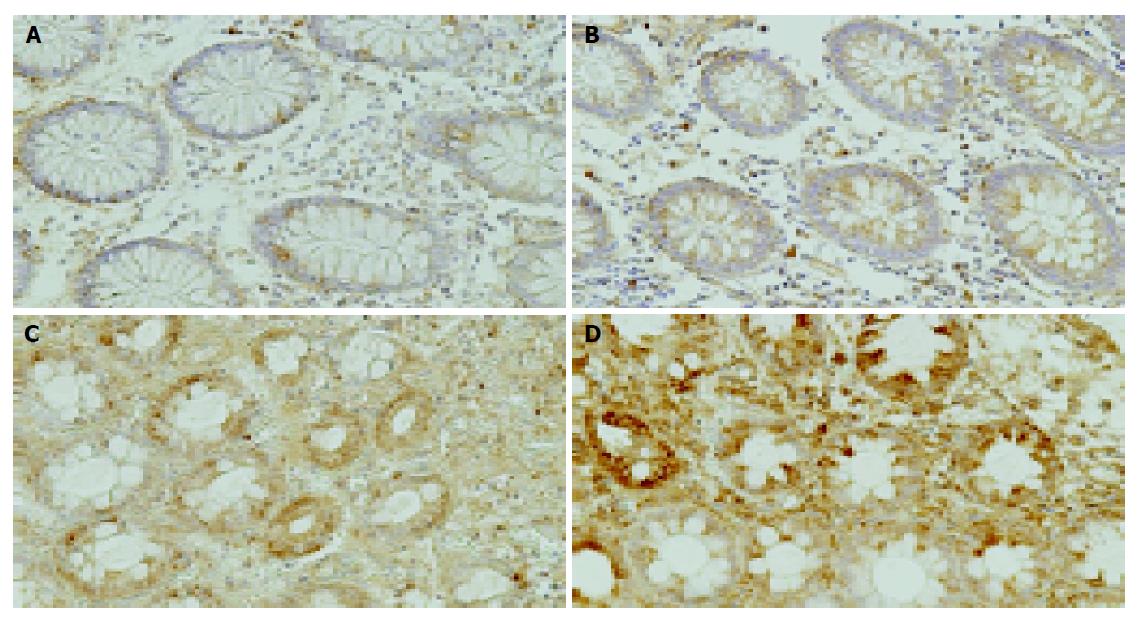
Weak HIF-1 alpha or VEGF immunoreactive cells were scattered in epithelial and interstitial cells in normal colon tissue (Figure 5A, B). In contrast, strong HIF-1 alpha or VEGF immunoreactive cells were diffusely seen in the epithelial and interstitial cells, including inflammatory cells in the ischemic colitis lesions (Figure 5C, D).
The first ischemic colitis report was a case report by Boley et al[15] in 1963, which founded the concept that was later accurately classified by Marston et al[16] in 1966. It has since become a well-recognized clinical entity. Most patients experience a sudden onset of mild, left-sided lower abdominal pain. Hematochezia may occur within 24 h. The most susceptible areas of the colon to ischemic damage are the “watershed regions”, which include the splenic flexure, descending and sigmoid colons. The endoscopic features are non-specific; erythematous and edematous mucosa, submucosal hemorrhages, and scattered areas of mucosal ulceration[1]. The well known pathological findings are degeneration, deciduation, necrosis, regeneration of the epithelial layer, congestion, fibrin thrombus in blood capillaries, leukocyte invasion in the lamina propria and bleeding, edema and exudates in the submucosal layer[4]. Ischemic crisis is believed to occur in ischemic colitis tissue. However, there is no precise pathophysiological examination for so-called “ischemic crisis” in this disease.
Semenza et al[5,17] discovered a new transcriptional factor; HIF-1 alpha in 1995. HIF-1 alpha is induced by tissue ischemia and promotes gene transcription[18]. HIF-1 alpha increases and activates some important proteins and enzymes, providing a strong defense against apoptosis subsequent to severe hypoxia. HIF-1 alpha has important roles in the production of erythropoietin in the kidneys, induction of anaerobic glycolysis, angiogenesis via VEGF and activation of myeloid cell-mediated inflammatory reactions[9,10]. Senger et al[19] reported a vascular permeability factor (VPF) in 1983 and Ferrara et al[20,23] independently identified a new angiogenic factor called VEGF in 1989. VEGF was the same as VPF, and it has been accepted that VEGF is one of the most important factors for angiogenesis[6]. VEGF has the functions of epithelial cell floating, proliferation and increasing the vascular permeability. VEGF is produced by the promoter signals from the HIF-1 alpha complex, secreted as a paracrine factor to extracellular space and binds to specific receptors, KDR/Flk-1 and Flt-1[12,13]. Then, the endothelial cell proliferation is promoted and finally angiogenesis is achieved under the hypoxic condition. In the present study, we demonstrated that two ischemic factors, HIF-1 alpha and VEGF, were constantly expressed in human colon tissue and were overexpressed in the ischemic colitis lesions. Our findings are the first molecular evidence of ischemia in ischemic colitis.
In addition, the overexpressed HIF-1 alpha and VEGF in the acute phase of ischemic colitis decreased to normal levels after 7 to 10 d. The colonoscopic findings also showed scarring in the lesion. These drastic changes suggest that when the ischemic crisis occurred, HIF-1 alpha and VEGF were induced immediately to prevent the expansion of tissue damage and to start quick repair to damaged tissue. Subsequently, these ischemic factors finished their role and their expression decreased to the ordinary state after the tissue recovered. This rapid change in the expression seems to differ from the continuous overexpression in inflammatory bowel disease[21,22].
We confirmed the ischemic crisis in ischemic colitis at the molecular level by analyzing the expression of HIF-1 alpha and VEGF. These ischemic factors may play an important role in the pathophysiology of ischemic colitis. We also demonstrated that HIF-1 alpha and VEGF were constantly expressed in normal human colon tissue including terminal ileum. The expression level of these factors was the same among regions of the colon, even though it is the descending colon where ischemic changes often occur. These ischemic factors were seen in epithelial cells and submucosal components. These results suggested that HIF-1 alpha and VEGF may play important roles in maintaining tissue integrity by regulating the delicate balance between proliferation and apoptosis. Further analysis of the function of these ischemic factors in the pathophysiology of the disease will be necessary in the future.
Edited by Li WZ Language Editor Elsevier HK
| 1. | Sreenarasimhaiah J. Diagnosis and management of intestinal ischaemic disorders. BMJ. 2003;326:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | MacDonald PH. Ischaemic colitis. Best Pract Res Clin Gastroenterol. 2002;16:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Hunt RH, Buchanan JD. Transient ischaemic colitis--colonoscopy and biopsy in diagnosis. J R Nav Med Serv. 1979;65:15-19. [PubMed] |
| 4. | Habu Y, Tahashi Y, Kiyota K, Matsumura K, Hirota M, Inokuchi H, Kawai K. Reevaluation of clinical features of ischemic colitis. Analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol. 1996;31:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4720] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 6. | Shibuya M. Role of VEGF-flt receptor system in normal and tumor angiogenesis. Adv Cancer Res. 1995;67:281-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 225] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987-7992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1593] [Cited by in RCA: 1643] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 8. | Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94:5667-5672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 308] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 820] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 10. | Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1528] [Cited by in RCA: 1621] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 11. | Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-4613. [PubMed] |
| 12. | Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1395] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 13. | Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349-9354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 760] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 14. | Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 407] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Boley SJ, Schwartz S, Lash J, Sternhill V. Reversible vascular occlusion of the colon. Surg Gynecol Obstet. 1963;116:53-60. [PubMed] |
| 16. | Marston A, Pheils MT, Thomas ML, Morson BC. Ischaemic colitis. Gut. 1966;7:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 278] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447-5454. [PubMed] |
| 18. | Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2734] [Cited by in RCA: 2678] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 20. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3536] [Cited by in RCA: 3554] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 21. | Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Kapsoritakis A, Sfiridaki A, Maltezos E, Simopoulos K, Giatromanolaki A, Sivridis E, Koukourakis MI. Vascular endothelial growth factor in inflammatory bowel disease. Int J Colorectal Dis. 2003;18:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 387] [Article Influence: 11.4] [Reference Citation Analysis (0)] |