Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1521
Revised: August 27, 2004
Accepted: September 1, 2004
Published online: March 14, 2005
AIM: To determine whether IFN-α is the agent that turns a slightly effective treatment (radiochemotherapy) into a potent therapy, we tested IFN-α for its synergistic properties.
METHODS: Eight pancreatic carcinoma cell lines were treated with the single agents and combinations of these. The role of IFN-α regarding a) direct inhibitory effects; b) radio and chemosensitizing effects; c) anti-angiogenic properties and d) enhancement of immunogenicity was investigated.
RESULTS: Our results show that IFN-α has direct inhibitory properties and some synergistic influence as determined by AnnexinV/PI stain and cell count. IFN-α is also able to prevent the increase in proliferation rate and VEGF secretion of CDDP resistant cells. Having taken the results from immunogenicity experiments together, we found cells that can be influenced by IFN-α but were less susceptible against T cells. Furthermore, high expression of MHC molecules, CD118, EGF-R and Fas was predictive for a good response.
CONCLUSION: In conclusion, IFN-α has direct cytotoxic effects, acts as a radiosensitizer and circumvents tumor cell-regrowth after CDDP treatment. These mechanisms may be responsible for the good clinical outcome of CapRI.
- Citation: Ma JH, Patrut E, Schmidt J, Knaebel HP, Büchler MW, Märten A. Synergistic effects of interferon-alpha in combination with chemoradiation on human pancreatic adenocarcinoma. World J Gastroenterol 2005; 11(10): 1521-1528
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1521.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1521
Carcinoma of the exocrine pancreas has an especially bad prognosis. The five-year survival rate is <1% with a median survival of 4-6 mo. Even after surgical intervention with a curative intention, the two-year survival rate is in specialized centers is at best 25%[1].
Investigators from the Virginia Mason Clinic have recently published data from a phase II trial of postoperative cisplatin (CDDP), 5-fluorouracil (5-FU), interferon alpha-2b (IFN-α), and external-beam radiation (RT) (that was) administered following pancreaticoduodenectomy. We termed this regimen CapRI for adjuvant treatment of pancreatic adenocarcinoma with ChemoRadioImmunotherapy. They treated 43 patients with mainly stage III tumors. Eighty-four percent had positive lymph nodes and 19% had positive cut margins. After a mean follow-up of 31.9 mo, 67% of the patients were still alive. Actuarial overall survival for the 1, 2, and 5-year survival rates were 95%, 64%, and 55% respectively. The treatment was quite toxic and 42% of the patients were hospitalized during the treatment, but there were no treatment-related deaths[2].
Various chemo and/or radiation regimens have been tested in small studies for treatment of adjuvant resected pancreatic carcinoma. Most of them use 5-FU or gemcitabine as the chemotherapeutic agent, sometimes in combination with other agents such as CDDP. These protocols are often combined with radiation[3]. The ESPAC-1 trial is the only trial to date that enrolled large numbers of patients (550 patients). The trial tested the hypothesis that chemoradiotherapy (40 Gy and weekly 5-FU) with or without 6 mo additional chemotherapy (5 FU, 425 mg/m2, day 1-5 and folinic acid, 20 mg/m2, d1-5, repeated monthly) provided an improvement in survival benefits compared to no adjuvant treatment. In a 2×2 factorial design, the 5-year survival rate for patients receiving chemoradiation was 10% and 19.6% without, and 21.1% for patients receiving chemotherapy and 8.4% without. The authors concluded from this that radiochemotherapy shows only limited success[4].
Comparing the data from CapRI and ESPAC-1, we hypothesize that IFN-α is the agent that turns a slightly effective treatment (chemoradiotherapy) into a potent therapy. Several mechanisms are described which might explain why IFN-α plays a potential role in combination therapies.
IFN-α belongs to the group of type I interferons, which are already being used in cancer therapy (e.g., malignant melanoma, renal cell carcinoma, hairy cell leukemia and chronic myeloid leukemia)[5,6]. IFN-α is produced by monocytes/macrophages, lymphoblastoid cells, fibroblasts and plasmacytoid dendritic cells[7]. Several other cell types are known to produce type I interferons after viral infections. IFN-α binds to the IFN-α receptor CD118; binding to the EBV-receptor CD21 is also described[8]. The IFN receptor is coupled to a Janus-family tyrosine kinase, which phosphorylates signal-transducing activators of transcription (STATs), which translocate to the nucleus where they activate the transcription of several different genes inducing the synthesis of host cell proteins that contribute to the inhibition of viral replication[9,10].
In addition to its anti-viral properties, IFN-α exhibits several other features that might be of interest especially for the use in combination treatments of cancer. Some of these features are: (1) direct inhibitory effects on tumor cell growth through prolongation of the cell cycle time of malignant cells, induction/inhibition of the expression of specific genes which lead to increased RNA degradation, inhibition of protein synthesis, down-regulation of oncogene expression, induction of tumor suppressor genes, and antagonism of the growth stimulatory effects of epidermal growth factor (EGF) and platelet-derived growth factor by down-regulation of cell surface receptors for the growth factors was reported[11,12]; (2) radio and chemosensitizing effects (described for 5-FU, cisplatin and dacarbazine)[13,14]; (3) anti-angiogenic properties. The latter is mainly due to the down-regulation of VEGF and by inhibiting the expression of pro-angiogenic molecules, basic fibroblast growth factor, and matrix metalloproteinase-9[15-17]; (4) enhancement of immunogenicity of tumors. This phenomenon is provoked by an increase of MHC class I expression which enhances immune recognition[12] and (5) modulation of the immune system. IFN-α plays an essential role in the differentiation, maturation and function of dendritic cells (DC), enhances the survival of T cells by expression of anti-apoptotic genes, induces the generation of CD8+ memory cells, enhances macrophage activities, and activates natural killer (NK) cells thus releasing cytokines[12,18-20].
Therefore, we investigated the influence of IFN-α in the CapRI-regimen in eight human ductal pancreatic carcinoma cell lines regarding direct cytotoxic effects of IFN-α, radio and/or chemosensitizing effects, anti-angiogenic properties and expression of MHC molecules and IFN-receptors.
Human pancreatic cancer cell lines ASPC-1, CAPAN-2, DAN-G, KciMoh-1, MiaPaCa-1, PANC-1, PatScl and PK 9 were obtained from ATCC, USA and cultured in RPMI 1 640 medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin (PAA laboratories GmbH, Austria). They were maintained at 37 °C under 50 mL/L CO2 in a humidified atmosphere.
Cells were subdivided into nine groups, each of which was treated with one of the following regimens almost in accordance with the protocol used in the CapRI scheme: (1) Untreated; (2) 5-FU 65 µg/mL continuously, d 1-5; f) 5-FU +IFN-α; (3) CDDP 3 µg/mL on d 1, for 60 min; (4) CDDP +IFN-α; (5) RT 1.8 Gy/d, d 1-5; (6) RT+IFN-α; (7) IFN-α 1000 U/mL, d 1, 3, and 5; (8) 5-FU+CDDP+ IFN-α+RT (CapRI).
Cells were investigated on day five of treatment. Apoptotic cells were detected on an Epics®XL.MCL flow cytometer (Beckman-Coulter, Krefeld, Germany) by AnnexinV staining using the AnnexinV/PI staining kit (BD PharmingenTM, Heidelberg, Germany) according to the manufacturer’s instructions. Cells were analyzed immediately after staining. Unstained cells were used to set up controls. Cell numbers of viable cells were determined by trypan blue exclusion.
Human pancreatic cancer cell lines were stained after treatment using various monoclonal antibodies directed against human cell surface antigens, including those against human HLA-ABC, HLA-DR, CD21, EGF-R, CD118, CD95 (Fas) and FasL (BD Biosciences, Heidelberg, Germany). For each flow cytometry measurement, gates for negative control were set to less than 2%. Data from at least 10000 cells were collected and analyzed. Negative controls consisted of the cells labeled with a corresponding isotype control. Mean fluorescence was normalized to mean fluorescence of the control antibodies. To distinguish whether an up-regulation of surface molecules has occurred or negative cells for that marker were preferentially killed; cells were stained before treatment, 24 h later analyzed, re-stained and re-analyzed. An increase in positive cells that was not seen in the control group was termed up-regulation. To distinguish between down-regulation of a specific surface marker and preferential killing of positive cells, we compared the percentage of marker and PI double-positive cells of cells with an adequate control.
The non-radioactive proliferation assay “EZ4U” kit (Biomedica, Vienna, Austria) was used according to the manufacturer’s instructions. Tumor cells were seeded out at a density of 2000 cells per well in triplicate in a 96-well plate. The proliferation rate was determined after 5 h of incubation as the amount of turnover of yellow tetrazolium salt to red formazan. Absorbance at 450 nm with 620 nm as a reference was measured with an ELISA reader; the absorbance of a medium blank was subtracted. The proliferation index was calculated by setting the proliferation of untreated cells to 1.
After a four-day treatment, the cells were seeded out at a density of 1×105 living cells in 2 mL. After 24 h, the cell culture supernatant was collected for each group and stored at -80 °C for further analysis. Amounts of VEGF were determined by usage of the DuoSet ELISA Development kit (R&D Systems, Inc., USA). The assay was performed in duplicate according to the manufacturer’s instructions.
Non-parametrical analysis (Mann-Whitney-U test) and parametrical correlation (Pearson) on SPSS 11.5 was used to analyze statistical significance. A P-value <0.05 was considered as significant.
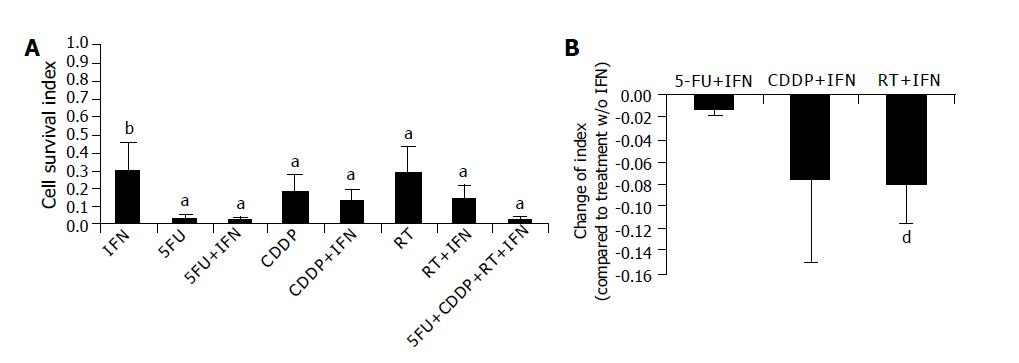
First, we determined the cell survival index (i.e., numbers of viable cells normalized to untreated cells [=1]) of eight cell lines by counting of viable cells after five-day treatment (Figure 1A). We found a statistically significant reduction of living cells after all treatments, which were more pronounced in 5-FU containing regimens. IFN-α alone decreased the cell survival index from 1 to 0.43±0.24 (P<0.002). With regard to radio or chemosensitizing effects, we found significant decrease of the index after adding IFN-α to radiotherapy (0.29±0.14 compared to 0.21±0.16; P<0.01; Figure 1B). A significant chemosensitizing effect was found in three out of eight cell lines (two times for 5-FU, one time for CDDP); however, it failed to be significant after taking the mean from all cell lines.
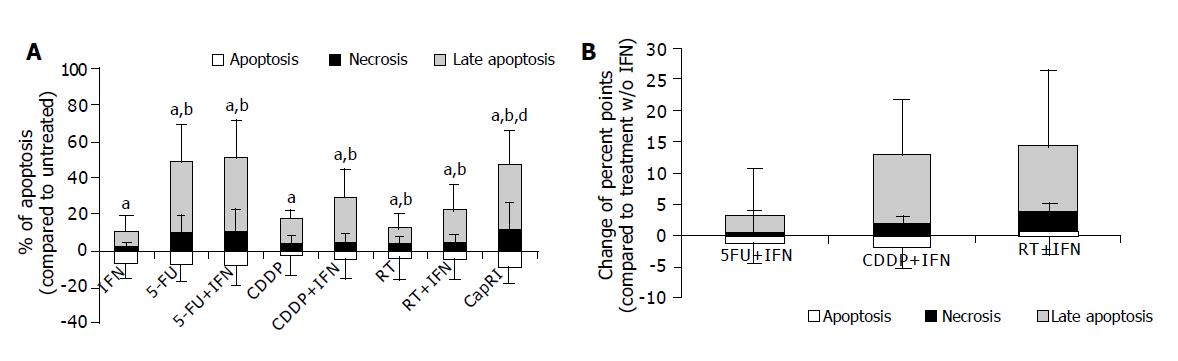
We determined apoptosis and necrosis of eight cell lines after five-day treatment by measuring AnnexinV and PI (Figure 2A). Results were normalized to values of untreated cells. We found a statistically significant increase of necrosis after all treatments. Late apoptosis (AnnexinV+/PI+) increased as well (significant for all but not for IFN-α treated cells). The sum of the dying cells was significantly increased after 5-FU and combination treatments as well as after CDDP+IFN-α treatment. Early apoptosis was not enhanced after treatments since it was determined after five days of treatment.
Regarding radio or chemosensitizing effects, we found a tendency towards sensitizing effects for adding of IFN-α to CDDP or radiotherapy. However, this was not significant (Figure 2B).
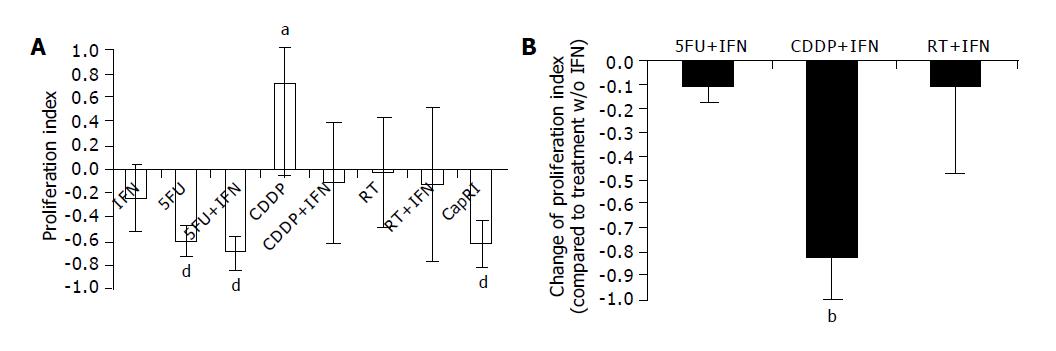
We determined the proliferation index (proliferation normalized to untreated cells [=1]) of eight cell lines by MTT assay after five-day treatment (Figure 3A). We found a statistically significant reduction in proliferation after treatments including 5-FU. IFN-α alone inhibited proliferation but not in a significant way. Interestingly, we observed an increase in proliferation after treatment with CDDP (P<0.005). This could be prevented by the addition of IFN-α (0.71±0.77 compared to -0.11±0.5; P<0.02; Figure 3B). Other radio or chemosensitizing effects could not be found.
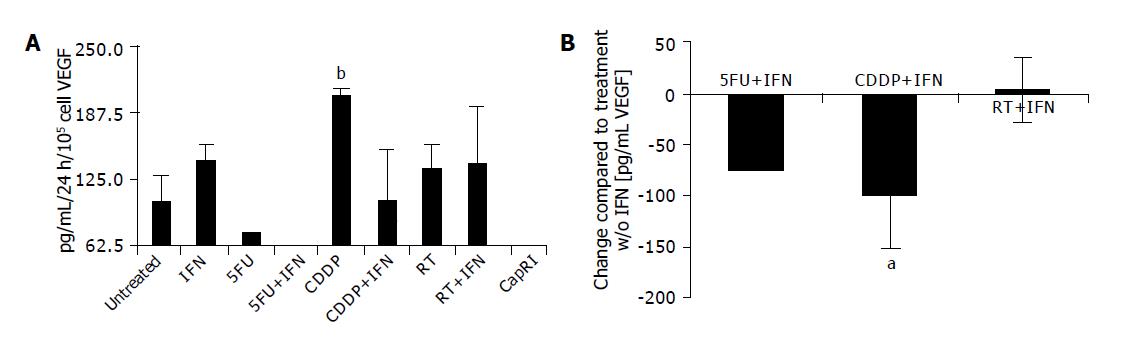
Just in three out of eight cell lines, detectable VEGF concentrations could be found in the supernatant. After treatment with 5-FU and combinations VEGF levels were mostly below the detection level (<62.5 pg/mL, Figure 4A). CDDP increases VEGF secretion (102.7±25.9 pg/mL/105 cells/24 h vs 204.7±6.4 after CDDP treatment, P<0.05). The addition of IFN-α to CDDP normalizes the amount of VEGF (104.2±47.8 pg/mL/105 cells/24 h, P<0.01; Figure 4B).
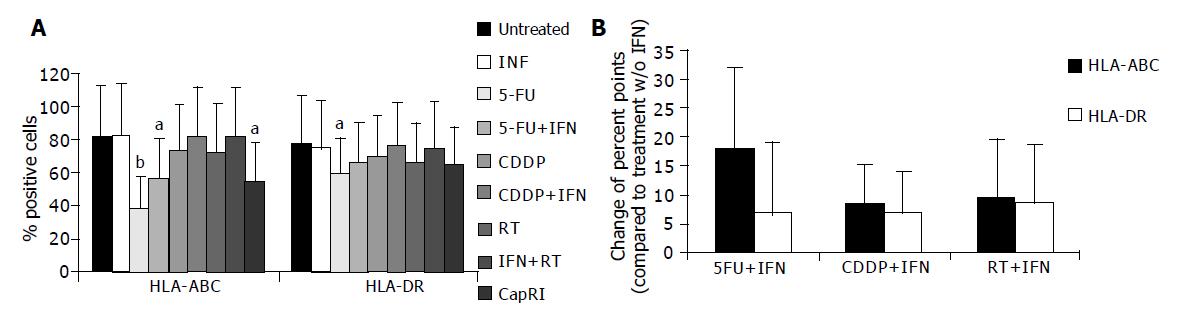
Next, we analyzed the influence of IFN-α on immunogenicity of tumor cells. Therefore, we determined constitutive and inducible MHC expression. Mean expression of MHC class I was significantly increased after IFN-α treatment (data not shown). However, we were not able to detect any influence of IFN-α at this concentration on the percentage of MHC positive cells (Figure 5A). After five-day treatment with 5-FU and combinations, we found a significant reduction in MHC-positive cells. This was due to down-regulation of MHC molecules. In five out of eight cell lines, this effect could be significantly softened when IFN-α is added to 5-FU. However, it failed to be significant after taking the mean from all cell lines (Figure 5B). There was a tendency towards higher MHC expression and mean fluorescence after adding IFN-α to chemo or radiotherapy; however, this was just significant for the mean fluorescence of HLA-ABC after adding IFN-α to CDDP.
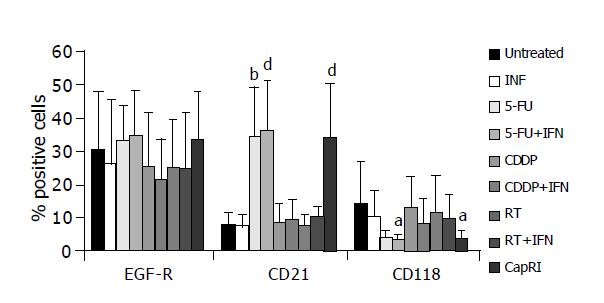
Furthermore, we analyzed expression of EGF-R and IFN-α-receptors (Figure 6). EGF-R expression remained mainly unchanged by treatment. CD118 (IFN-R) was down-regulated after treatment, this was significant for treatment with 5-FU+IFN-α and CapRI scheme (13.9±12.8% on untreated cells vs 3.2±1.8% or 3.5±2.7%, respectively). CD21, which is described to act as an IFN-α receptor was only slightly expressed on untreated cells and was significantly up-regulated after treatment with 5-FU and combinations (7.8±3.5% on untreated cells vs. approximately 35% after treatment). IFN-α had no synergistic effect for any treatment. Furthermore, we observed a decrease of Fas-positive cells after treatment (data not shown).
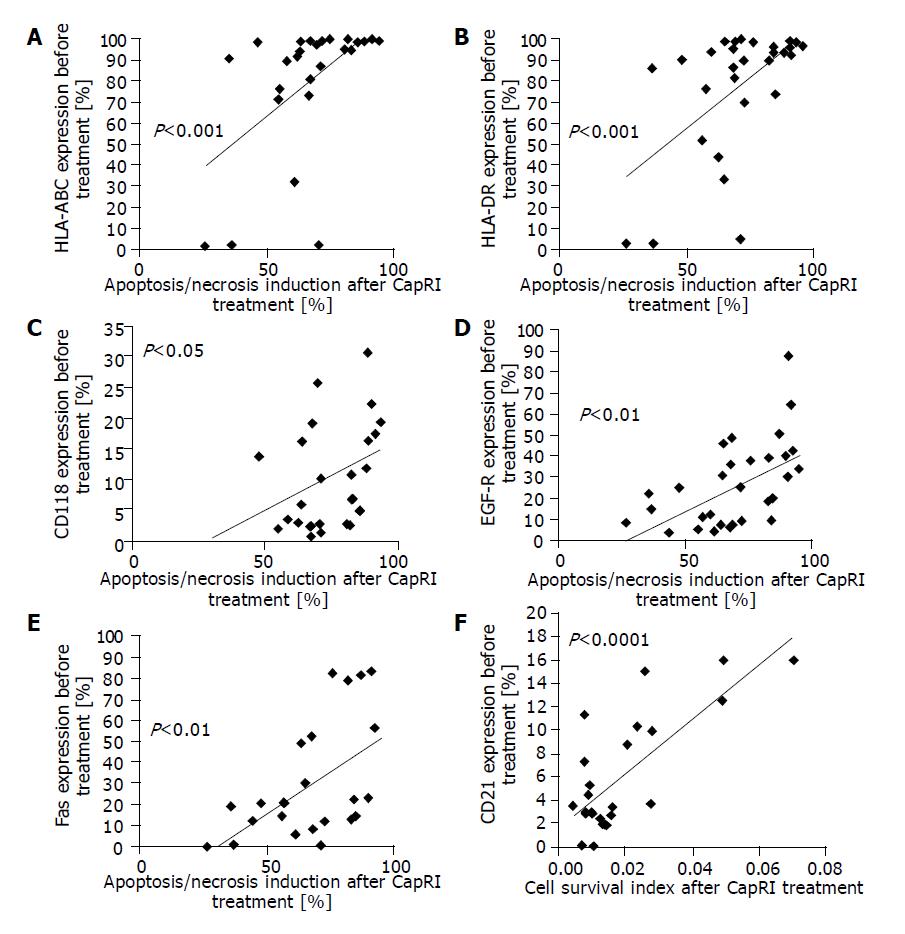
Since we were interested in potentially predictive markers for treatment responsiveness, we analyzed expression of several markers on untreated cells for correlation with apoptosis/necrosis induction, decrease of cell survival and anti-proliferative effects. All parameters behaved in a similar way, mostly in a significant way (data from analysis of proliferation index correlated with induction of apoptosis/necrosis that correlated with late apoptosis, which in turn correlated with cell survival index).
We found a significant correlation between expression of MHC molecules, CD118, Fas and EGF-R on untreated cells with apoptosis/necrosis induction after treatment with the CapRI scheme (Figure 7A-E). Low expression of CD21 correlated with a low cell survival index (i.e., with low numbers of viable cells, Figure 7F).
Next, we analyzed the treatment-response-pattern of parameters to define surrogate markers (Table 1). With regard to MHC molecules, IFN-receptors, EGF-receptor and Fas, almost all cell lines reacted in a similar way to the different treatments. The correlation was positive except for EGF-R and CD21 where we observed a negative correlation. Interestingly, treatments inducing apoptosis/necrosis also induce a decrease in MHC, CD118 and Fas-positive cells and an increase in CD21-positive cells. Treatments that inhibit proliferation also induce an increase of CD21-positive cells as well as a decrease of CD118 and Fas-positive cells and of VEGF secretion. Apoptosis/necrosis induction correlated with the anti-proliferative effect as well as with the number of surviving cells.
| HLA-ABC | HLA-DR | HLA-ABC mean | HLA-DR mean | CD 21 | CD 118 | EGF-R | Fas | FasL | VEGF | Proliferation index | Apoptosis/necrosis | Cell survival index | |
| HLA-ABC | |||||||||||||
| Pearson’s correlation | 0.928 | 0.783 | 0.777 | -0.907 | 0.790 | -0.769 | 0.887 | 0.867 | -0.825 | ||||
| P | 0.001 | 0.012 | 0.014 | 0.001 | 0.011 | 0.015 | 0.001 | 0.002 | NS | NS | 0.006 | NS | |
| HLA-DR | |||||||||||||
| Pearson’s | 0.928 | 0.71 | 0.743 | -0.753 | 0.762 | 0.782 | -0.705 | ||||||
| P | 0.001 | 0.032 | 0.022 | 0.019 | NS | NS | 0.017 | 0.013 | NS | NS | 0.034 | NS | |
| HLA-ABC mean | |||||||||||||
| Pearson’s | 783 | 0.71 | 0.939 | -0.718 | -0.892 | ||||||||
| P | 0.012 | 0.032 | 0.001 | 0.029 | NS | 0.001 | NS | NS | NS | NS | NS | NS | |
| HLA-DR mean | |||||||||||||
| Pearson’s | 0.777 | 0.743 | 0.939 | -0.738 | |||||||||
| P | 0.014 | 0.022 | 0.001 | NS | NS | 0.023 | NS | NS | NS | NS | NS | NS | |
| CD 21 | |||||||||||||
| Pearson’s | -0.907 | -0.753 | -0.718 | -0.926 | 0.85 | -0.976 | -0.957 | -0.810 | 0.915 | ||||
| P | 0.001 | 0.019 | 0.029 | NS | 0.001 | 0.004 | 0.001 | 0.001 | NS | 0.008 | 0.001 | NS | |
| CD 118 | |||||||||||||
| Pearson’s | 0.79 | -0.926 | 0.966 | 0.946 | 0.596 | 0.898 | -0.936 | 0.718 | |||||
| P | 0.011 | NS | NS | NS | 0.001 | NS | 0.0001 | 0.0001 | 0.158 | 0.001 | 0.001 | 0.029 | |
| EGF-R | |||||||||||||
| Pearson’s | -0.769 | -0.892 | -0.738 | 0.850 | -0.764 | -0.758 | |||||||
| P | 0.015 | NS | 0.001 | 0.023 | 0.004 | NS | 0.017 | 0.018 | NS | NS | NS | NS | |
| Fas | |||||||||||||
| Pearson’s | 0.887 | 0.762 | -0.976 | 0.966 | -0.764 | 0.985 | 0.888 | -0.922 | |||||
| P | 0.001 | 0.017 | NS | NS | 0.0001 | 0.0001 | 0.017 | 0.001 | NS | 0.001 | 0.001 | NS | |
| FasL | |||||||||||||
| Pearson’s | 0.867 | 0.782 | -0.957 | 0.946 | -0.758 | 0.985 | 0.875 | -0.886 | 0.675 | ||||
| P | 0.002 | 0.013 | NS | NS | 0.001 | 0.001 | 0.018 | 0.001 | NS | 0.002 | 0.001 | 0.046 | |
| VEGF | |||||||||||||
| Pearson’s 0.8 | |||||||||||||
| P | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.031 | NS | NS | |
| Proliferation index | |||||||||||||
| Pearson’s | -0.81 | 0.898 | 0.888 | 0.875 | 0.800 | ||||||||
| P | NS | NS | NS | NS | 0.008 | 0.001 | NS | 0.001 | 0.002 | 0.031 | NS | NS | |
| Apoptosis/necrosis | |||||||||||||
| Pearson’s | -0.825 | -0.705 | 0.915 | -0.936 | -0.922 | -0.886 | -0.721 | -0.748 | |||||
| P | 0.006 | 0.034 | NS | NS | 0.001 | 0.001 | NS | 0.001 | 0.001 | NS | 0.028 | 0.020 | |
| Cell survival index | |||||||||||||
| Pearson’s | 0.718 | 0.675 | -0.748 | ||||||||||
| P | NS | NS | NS | NS | NS | 0.029 | NS | NS | 0.046 | NS | NS | 0.020 | |
Carcinoma of the exocrine pancreas has an especially bad prognosis. Treatment after adjuvant resection prolongs the survival, but so far has failed to produce long-lasting benefits. Results from a phase II trial, where chemoradiotherapy was combined with IFN-α are very encouraging (55% 5-year survival compared to 10% in the ESPAC-1 trial).
Comparing the data from the CapRI and the ESPAC-1 trials, we hypothesize that IFN-α is the agent that turns a slightly effective treatment (radiochemotherapy) into a potent therapy. Several mechanisms are described that might explain why IFN-α could play a potential role in combination therapies. Here, we focused on the described mechanism of a) direct inhibitory effects on tumor cell growth[12]; b) radio- and chemosensitizing effects[13,14]; c) anti-angiogenic properties (as far as possible)[15-17] and d) enhancement of immunogenicity of tumors[12].
With regard to direct inhibitory effects we observed cytotoxic effects of IFN-α. A synergistic effect of IFN-α was observed after the addition to radiotherapy but not to chemotherapy. It is reported that IFN-α down-regulates EGF-R on renal cell carcinoma and breast cancer cells after 7 d of incubation and that this consequently results in cell growth inhibition[11,12]. With a shorter incubation period, we were not able to confirm these data for pancreatic carcinoma. IFN-α treatment resulted only in a partial down-regulation of EGF-R. Similar observation was seen regarding the apoptosis rate after five days of treatment. In all cell lines, we found that only a few cells were in early apoptosis, some necrotic cells and the majority of the cells were in late apoptosis. Since there were many manipulations during the treatment period as well as for the untreated control group (e.g., daily harvesting for radiation), we had a high early apoptosis rate in untreated cells. But only treated cells showed remarkably high rates of late apoptosis and necrosis. This effect was not so pronounced in IFN-α treated cells. There was a tendency towards a synergistic effect of IFN-α; however, it was not significant. Taken together, we must conclude that IFN-α has direct inhibitory properties and shows limited synergistic influence.
Next, we analyzed the proliferative capacity of surviving cells after five days of treatment. Interestingly, we found a significant increase in proliferation of CDDP resistant cells. This is in accordance with data from various groups who observed an accelerated tumor growth after chemotherapy[21,22] in vitro as well as in vivo. IFN-α on its own has a slight inhibitory effect on proliferation rate, but was able to circumvent the accelerated tumor cell growth after CDDP treatment. Best results were seen again for treatments including 5-FU with no synergistic effect of IFN-α. Similar results could be observed for the secretion of VEGF. We tested this parameter although we are aware of the problems of this surrogate marker for in vitro analysis of anti-angiogenic effects. Only 3/8 cell lines secreted measurable amounts of VEGF. However, this significantly increased after treatment with CDDP; an effect which could again be circumvented by the addition of IFN-α. As Tannock concluded his article about ‘Repopulation of tumor cells between cycles of chemotherapy: a neglected factor’ with the sentence ‘Biological agents with rapid onset and short duration of action’, which can selectively inhibit tumor-cell repopulation, administered between cycles of chemotherapy, might improve the therapeutic index[22]. Perhaps, IFN-α is one of these biological agents.
We also looked at effects of IFN-α on immunogenicity of the tumor cells. As expected, we observed an increase in MHC molecules on the surface of the tumor cells after treatment with IFN-α. However, cell lines completely deficient for MHC did not change. Interestingly, we observed a significant decrease of MHC class I-positive cells after treatment with 5-FU and combinations. Our findings were different from what has been reported[23-25] where an increase in MHC class I on various epithelial tumor cells after treatment with 5-FU was described. We can only speculate why we observed an opposite effect. Perhaps the continuous culture in the presence of high-dosed 5-FU over five days without any synergistic acting agent, such as levamisole, is responsible for our observations. This is supported by the observation that adding IFN-α to 5-FU at least diminishes the down-regulation and that treatment with 5-FU over 24 h had no effect. AbdAlla et al[24] described that 5-FU increase the steady state level of HLA class I mRNAs by about 80% but stated also a reduction of 20-57% in RNA synthesis. This could explain why after a ‘long-time’ treatment, MHC molecules were down-regulated.
As another feature of immunogenicity, we investigated the expression of IFN-R. Pancreatic tumor cells were always able to bind IFN-α. Although 5-FU down-regulates CD118, it provokes an increase of CD21, which acts as well as an IFN-R. This up-regulation may be due to histone acetyltransferase activity of 5-FU that allows specific transcription factors access to the cognate DNA binding site[26]. From the available results for immunogenicity, we observed cells that can be addressed by IFN-α but have a moderate reduction in T cell susceptibility.
We were also interested in predictive markers for response to the CapRI scheme. Therefore, we used apoptosis/necrosis induction, low cell survival index and negative proliferation index as parameters for good response; all parameters behaved very similar. For our in vitro experiments, we found a correlation between high expression in MHC molecules, CD118, EGF-R and Fas and apoptosis/necrosis induction. We do not know why MHC-positive cells are more susceptible to the treatment but the better response of Fas, CD118 and EGF-R-positive cells make sense. Fas-induced cell death may play a role in this treatment and CD118 expression is necessary for effects by IFN-α that is described to inhibit EGF-stimulated cell growth and reduce EGF binding[11]. Furthermore, we found a very similar reaction pattern to the different treatments with regard to most markers. Even if they are not dependent on each other, they could be used as surrogate markers.
In conclusion, we demonstrated that IFN-α has direct inhibitory properties with limited synergistic influence and that it reverts to the enhanced proliferation rate and VEGF secretion after CDDP treatment and the decreased MHC class I expression after 5-FU treatment. These results may be responsible for the positive outcome of the CapRI scheme. However, we hypothesize that the activation of the immune system by IFN-α plays a very important role in this treatment scheme, too. Further studies are started to investigate this mechanism.
We thank Karin Steybe for excellent technical assistance and Moustafa Kebbewar for proofreading of the manuscript.
Co-first-authors: Emilia Patrut and Jan Schmidt
Edited by Li WZ Language Editor Elsevier HK
| 1. | Raraty MG, Magee CJ, Ghaneh P, Neoptolemos JP. New techniques and agents in the adjuvant therapy of pancreatic cancer. Acta Oncol. 2002;41:582-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Tsai JY, Iannitti DA, Safran H. Combined modality therapy for pancreatic cancer. Semin Oncol. 2003;30:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1908] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 5. | Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002;29:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA. 1994;91:1198-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 505] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Delcayre AX, Salas F, Mathur S, Kovats K, Lotz M, Lernhardt W. Epstein Barr virus/complement C3d receptor is an interferon alpha receptor. EMBO J. 1991;10:919-926. [PubMed] |
| 9. | Mogensen KE, Lewerenz M, Reboul J, Lutfalla G, Uzé G. The type I interferon receptor: structure, function, and evolution of a family business. J Interferon Cytokine Res. 1999;19:1069-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Domanski P, Witte M, Kellum M, Rubinstein M, Hackett R, Pitha P, Colamonici OR. Cloning and expression of a long form of the beta subunit of the interferon alpha beta receptor that is required for signaling. J Biol Chem. 1995;270:21606-21611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 180] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Iacopino F, Ferrandina G, Scambia G, Benedetti-Panici P, Mancuso S, Sica G. Interferons inhibit EGF-stimulated cell growth and reduce EGF binding in human breast cancer cells. Anticancer Res. 1996;16:1919-1924. [PubMed] |
| 12. | Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, Walter MR, Nagabhushan TL, Trotta PP, Pestka S. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58:2489-2499. [PubMed] |
| 13. | Kurzrock R, Talpaz M, Guttermann J. Interferons: Clinical applications. Philadelphia: Lippincott; 1991; . |
| 14. | Holsti LR, Mattson K, Niiranen A, Standertskiöld-Nordenstam CG, Stenman S, Sovijärvi A, Cantell K. Enhancement of radiation effects by alpha interferon in the treatment of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1987;13:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Decatris M, Santhanam S, O'Byrne K. Potential of interferon-alpha in solid tumours: part 1. BioDrugs. 2002;16:261-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Solorzano CC, Hwang R, Baker CH, Bucana CD, Pisters PW, Evans DB, Killion JJ, Fidler IJ. Administration of optimal biological dose and schedule of interferon alpha combined with gemcitabine induces apoptosis in tumor-associated endothelial cells and reduces growth of human pancreatic carcinoma implanted orthotopically in nude mice. Clin Cancer Res. 2003;9:1858-1867. [PubMed] |
| 17. | Wang L, Wu WZ, Sun HC, Wu XF, Qin LX, Liu YK, Liu KD, Tang ZY. Mechanism of interferon alpha on inhibition of metastasis and angiogenesis of hepatocellular carcinoma after curative resection in nude mice. J Gastrointest Surg. 2003;7:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64:358-367. [PubMed] |
| 19. | Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999;93:1980-1991. [PubMed] |
| 20. | Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 600] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 21. | El Sharouni SY, Kal HB, Battermann JJ. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br J Cancer. 2003;89:2184-2189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Davis AJ, Tannock JF. Repopulation of tumour cells between cycles of chemotherapy: a neglected factor. Lancet Oncol. 2000;1:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Correale P, Aquino A, Giuliani A, Pellegrini M, Micheli L, Cusi MG, Nencini C, Petrioli R, Prete SP, De Vecchis L. Treatment of colon and breast carcinoma cells with 5-fluorouracil enhances expression of carcinoembryonic antigen and susceptibility to HLA-A(*)02.01 restricted, CEA-peptide-specific cytotoxic T cells in vitro. Int J Cancer. 2003;104:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | AbdAlla EE, Blair GE, Jones RA, Sue-Ling HM, Johnston D. Mechanism of synergy of levamisole and fluorouracil: induction of human leukocyte antigen class I in a colorectal cancer cell line. J Natl Cancer Inst. 1995;87:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Ohtsukasa S, Okabe S, Yamashita H, Iwai T, Sugihara K. Increased expression of CEA and MHC class I in colorectal cancer cell lines exposed to chemotherapy drugs. J Cancer Res Clin Oncol. 2003;129:719-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Zabel MD, Weis JH. Cell-specific regulation of the CD21 gene. Int Immunopharmacol. 2001;1:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |