Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1452
Revised: September 2, 2004
Accepted: November 26, 2004
Published online: March 14, 2005
AIM: To explore the impact of prolonged fraction dose-delivery time modeling intensity-modulated radiation therapy (IMRT) on cell killing of human hepatocellular carcinoma (HCC) HepG2 and Hep3B cell lines.
METHODS: The radiobiological characteristics of human HCC HepG2 and Hep3b cell lines were studied with standard clonogenic assays, using standard linear-quadratic model and incomplete repair model to fit the dose-survival curves. The identical methods were also employed to investigate the biological effectiveness of irradiation protocols modeling clinical conventional fractionated external beam radiotherapy (EBRT, fraction delivery time 3 min) and IMRT with different prolonged fraction delivery time (15, 30, and 45 min). The differences of cell surviving fraction irradiated with different fraction delivery time were tested with paired t-test. Factors determining the impact of prolonged fraction delivery time on cell killing were analyzed.
RESULTS: The α/β and repair half-time (T1/2) of HepG2 and Hep3b were 3.1 and 7.4 Gy, and 22 and 19 min respectively. The surviving fraction of HepG2 irradiated modeling IMRT with different fraction delivery time was significantly higher than irradiated modeling EBRT and the cell survival increased more pronouncedly with the fraction delivery time prolonged from 15 to 45 min, while no significant differences of cell survival in Hep3b were found between different fraction delivery time protocols.
CONCLUSION: The prolonged fraction delivery time modeling IMRT significantly decreased the cell killing in HepG2 but not in Hep3b. The capability of sub-lethal damage repair was the predominant factor determining the cell killing decrease. These effects, if confirmed by clinical studies, should be considered in designing IMRT treatments for HCC.
- Citation: Zheng XK, Chen LH, Yan X, Wang HM. Impact of prolonged fraction dose-delivery time modeling intensity-modulated radiation therapy on hepatocellular carcinoma cell killing. World J Gastroenterol 2005; 11(10): 1452-1456
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1452.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1452
With the popularization of intensity-modulated radiation therapy (IMRT), an irradiation technique developed to improve target dose conformity and normal tissue sparing[1-4], more and more patients with hepatocellular carcinoma (HCC) would receive radiotherapy[5]. IMRT optimized the physical dose distribution of radiotherapy, which thereby could enhance the tumor local control and lower the radiation-induced hepatitis. However, the radiobiological effectiveness of IMRT might be different from conventional external beam radiation therapy (EBRT) especially considering the prolonged fraction delivery time in IMRT. IMRT delivers dose, either dynamically or statically (e.g., step-and-shoot), using many beam apertures (segments) that are shaped with multileaf collimator[1,4,6]. It takes a much longer time to deliver a single fraction dose with IMRT than EBRT. Generally, EBRT takes about 2-5 min to deliver a single fractional dose, whereas IMRT with static delivery requires 15-45 min to deliver the same fractional dose. According to radiobiological theory, cell killing tends to decrease with fraction delivery time increasing because of ongoing sublethal damage repair (SLDR) processes during dose delivery. Wang et al[7] calculated the cell-killing efficiency of simulated and clinical IMRT plans with the generalized linear-quadratic (LQ) model, which indicated that fraction delivery times in the range of 15-45 min may significantly decrease tumor cell killing and may have a significant impact on treatment outcome for tumors with a low α/β ratio and short repair half-time (T1/2). However, such calculation lacks confirmation of studies in vitro. To clarify the impact of prolonged fraction delivery time in IMRT on tumor cell killing, more detailed studies in vitro are required.
In this study, we attempt to ascertain the impact of prolonged fraction delivery time modeling IMRT on survival of human HCC cell lines HepG2 and Hep3B, so as to provide radiobiological basis for optimizing IMRT plans for this disease.
Human HCC cell lines including HepG2 and Hep3b were employed in this study. Both cell lines were cultured in plastic flasks at 37 °C in a humidified atmosphere of 50 mL/L CO2 and 95% air with the 1640 medium containing 10-15% fetal calf serum with 100 U/mL penicillin and 100 μg/mL streptomycin. Results of regular tests for mycoplasma contamination were negative. When they become confluent, cells were sub-cultured (1:4 dilution). Exponentially growing cells were used for experiments.
Immediately prior to irradiation, single-cell suspension was prepared by trypsination and cell number was counted using a hemocytometer. Cells were then seeded in varying amounts onto 6-well tissue culture dishes with 1640 medium. Three parallel samples were set at each radiation dose of various irradiation schedules.
Irradiation was carried out at room temperature using a 6-MeV X-ray. To learn the radiobiological characteristics, doses of 0 Gy, 1 Gy, 2 Gy, 4 Gy, 6 Gy, 8 Gy and 10 Gy were given as single, continuous doses at a dose rate of 3.2 Gy/min for generating standard dose-survival curves and acquiring a variety of mathematic model parameters. To achieve the T1/2, doses of 0 Gy, 1 Gy, 2 Gy, 4 Gy, 6 Gy, 8 Gy and 10 Gy were given as single, continuous doses at a dose rate of 0.066 Gy/min. To compare the cell killing effectiveness of fraction delivery time modeling EBRT and IMRT, fractionated irradiation of 0 Gy, 1 Gy×1, 2 Gy×1, 2 Gy×2, 2 Gy×3, 2 Gy×4 and 2 Gy×5, were given with one fraction per day just like clinical dose-time-fractionation pattern. In irradiation modeling EBRT, the fraction delivery time was 3 min, with two 1.18 min intervals modeling 3 portals irradiation. In irradiation modeling IMRT with different fraction delivery times, each fraction dose was given in seven equal sub-fractions; the total fraction delivery time was 15, 30 or 45 min.
Standard clonogenic assays were used to acquire the standard dose-survival curves of HepG2 and Hep3b and to determine the effect of irradiation modeling EBRT and IMRT with fraction delivery time of 15, 30 and 45 min. Cells plated in 6-well tissue culture dishes were incubated in an undisturbed state for 10 d. Cell fixation and staining used methanol and 0.5% crystal violet in deionized water and colony counts were performed by visual inspection. A colony was defined as 50 or more cells. Colony plating efficiency was calculated to be the number of viable nucleated cells plated and expressed as a percentage. The surviving fraction at each dose of each irradiation protocols was determined by dividing the plating efficiency of the irradiated cells by that of the untreated control. All data points were the mean results of experiments.
Dose-survival curves for each experiment were constructed by semi-logarithmically plotting the mean surviving fractions as a function of irradiation dose. The data were analyzed, and survival curves were plotted following the standard linear-quadratic model [S = exp (-αD - βD2)] or incomplete repair model [S = exp (-αD - βgD2)][8] using GraphPad Prism 4.0 software (GraphPad Software, Inc., USA). α and β resulted from the best fitting survival curves and were used to calculate surviving fraction at 2 Gy (SF2).
Differences of surviving fraction treated with different irradiation protocols were tested with paired t-test. Statistical significance was assumed when P<0.05.
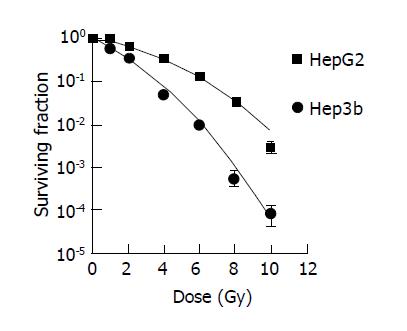
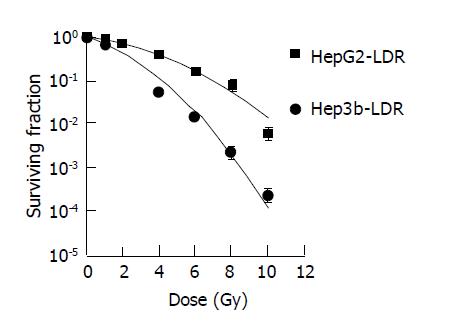
Standard dose-survival curves of HepG2 and Hep3b fitted with the standard LQ model are shown in Figure 1. The survival curves with irradiation at a low dose rate of 0.066 Gy/min fitted with incomplete repair model to acquire the T1/2 of both cell lines were shown in Figure 2. The radiobiological characteristics of both cell lines described with the parameters of the mathematic models are listed in Table 1.
| Cell | Parameters | |||||
| SF2read (%) | SF2est (%) | α (Gy-1) | β (Gy-2) | α/β (Gy) | T1/2(min) | |
| HepG2 | 89 | 67.6 | 0.118 | 0.038 | 3.1 | 22 |
| Hep3b | 60 | 34.6 | 0.413 | 0.056 | 7.4 | 19 |
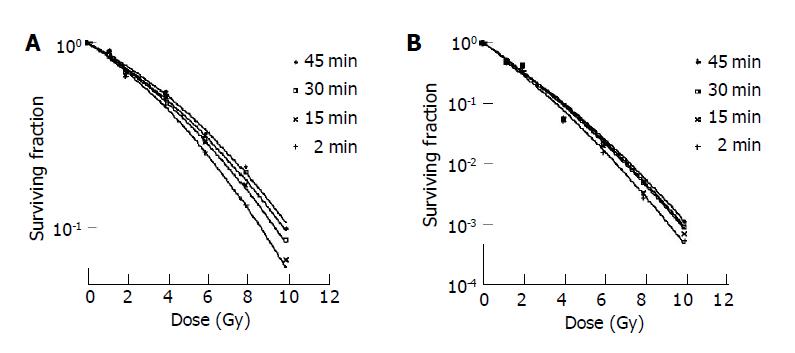
Surviving fraction of HepG2 and Hep3b irradiated modeling fractionated EBRT of 0Gy, 1Gy×1, 2Gy×1, 2Gy×2, 2Gy×3, 2Gy×4 and 2Gy×5, as well as modeling IMRT given in seven equal sub-fractions per fraction delivered within a total fraction delivery time of 15, 30 or 45 min are listed in Table 2. The dose-survival curves of both cell lines with different fractionated irradiation schedules fitted with standard LQ model are shown in Figure 3. The surviving fractions of HepG2 irradiated modeling IMRT with different fraction delivery times were significantly higher than irradiated modeling EBRT (P<0.05), and the cell irradiated modeling IMRT with longer fraction delivery time has a significant higher survival than that with shorter fraction delivery time (P<0.05). No significant survival differences of Hep3b were found between different fraction delivery time protocols (P>0.05) (Table 3).
| Dose | Survival fraction of HepG2 and Hep3b | |||||||
| Modeling EBRT-03’ | Modeling IMRT-15’ | Modeling IMRT-30’ | Modeling IMRT-45’ | |||||
| HepG2 | Hep3b | HepG2 | Hep3b | HepG2 | Hep3b | HepG2 | Hep3b | |
| 0.0 Gy | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1.0 Gy×1 | 0.87 | 0.48 | 0.88 | 0.48 | 0.88 | 0.48 | 0.91 | 0.48 |
| 2.0 Gy×1 | 0.6636 | 0.3559 | 0.6747 | 0.4123 | 0.7012 | 0.4389 | 0.7133 | 0.45 |
| 2.0 Gy×2 | 0.4669 | 0.0507 | 0.5123 | 0.0546 | 0.5181 | 0.055 | 0.5498 | 0.059 |
| 2.0 Gy×3 | 0.2578 | 0.055 | 0.2906 | 0.02 | 0.3006 | 0.0208 | 0.3236 | 0.023 |
| 2.0 Gy×4 | 0.1321 | 0.0027 | 0.17 | 0.0033 | 0.1987 | 0.0049 | 0.2137 | 0.0051 |
| 2.0 Gy×5 | 0.0547 | 0.0005 | 0.0671 | 0.0007 | 0.0851 | 0.0009 | 0.0996 | 0.0011 |
| Fraction delivery time protocols | HepG2 | Hep3b | ||
| t | P | t | P | |
| EBRT-03’vs IMRT-15’ | -3.308 | 0.016 | -1.199 | 0.276 |
| EBRT-03’vs IMRT-30’ | -3.909 | 0.008 | -1.179 | 0.283 |
| EBRT-03’vs IMRT-45’ | -4.814 | 0.003 | -1.24 | 0.261 |
| IMRT-15’vs IMRT-30’ | -2.823 | 0.03 | -1.133 | 0.301 |
| IMRT-15' vs IMRT-45’ | -5.686 | 0.001 | -1.301 | 0.241 |
| IMRT-30’vs IMRT-45’ | -4.308 | 0.005 | -1.644 | 0.151 |
This study demonstrated that the prolonged fraction delivery time modeling IMRT decreased the cell killing of HepG2; the cell killing decreased more pronouncedly with the fraction delivery time being prolonged from 15 min to 45 min. These phenomena, however, were not obvious in Hep3b.
The intrinsic reason for increasing of the cell survival treated with IMRT like protocols is the ongoing SLDR processes during dose delivery. Irradiated tumor cells may be lethally or not lethally damaged. Cells that are not lethally damaged may undergo repair. SLDR is an important type of damage repair that is defined as the enhancement in survival when a dose of radiation is separated over a period time. Generally, SLDR experiments divide a single dose into two relatively equal doses spaced at variable time intervals. Elkind et al investigated this phenomenon in great detail[9,10]. An enhancement in survival after two doses separated in time was observed. This enhancement in survival was due to SLDR.
The survival increasing of cells irradiated with a prolonged fraction delivery time is mainly associated with the capacity and rate of the SLDR during the dose delivery as well as the fraction dose delivery time.
The capacity of a cell to undergo SLDR, which is associated with the intrinsic radiobiological characteristics of the cell, may be represented by the quadratic term in LQ model. A cell with a small α/β is considered having a large ability to undergo SLDR. Most human tumor cell lines studied in vitro have a relatively small ability to undergo SLDR[11-15]. Yet, a large capacity for SLDR has been reported for some human tumor cell lines[12,16-20]. According to this study, HepG2 has a relatively large capacity for SLDR with a α/β of 3.1, which is much lower than what was expected, while Hep3b has a smaller capacity for SLDR with a α/β of 7.4.
The rate of SLDR can be represented with T1/2. Apparently, cells with short T1/2 have more repairs during a certain fraction delivery time. For human tumor cell lines, the characteristic T1/2 ranges from a few minutes to several hours[21-23]. In a review article, Steel et al[21] pointed out that the repair time for many tumors appears different when measured from a split-dose experiment vs a low-dose-rate exposure. They attributed this difference to the presence of two or more repair components. Others have confirmed that non-exponential or multi-exponential SLDR kinetics are involved in cell killing[24-28]. In split-dose survival experiments, the fast and slow rates of SLDR kinetics can be reasonably approximated by a single (average) first-order repair term. In low-dose-rate experiments, cell killing is more sensitive to the fast repair component. For fraction delivery times in the range of 15 to 45 min (i.e., comparable to IMRT treatment times), the fast repair component is important and the slow repair component has little impact on cell killing. Brenner and Hall[22] have compiled in vitro data on the T1/2 of human cancer cell lines under low-dose-rate exposure conditions. They have found that the most probable T1/2 is approximately 20 min. For prostate cancer, Wang et al[17] used clinical data to derive a T1/2 of 16 min. In this study, the T1/2 of HepG2 and Hep3b were 22 and 19 min respectively. They are just within the general fraction delivery time of IMRT (15-45 min). Although cells, with shorter T1/2 (Hep3b), could not be proved as having more SLDR than that with longer T1/2 (HepG2) in this study for the much larger SLDR capability of HepG2 than Hep3b, the importance of SLDR rate should not be neglected only if the T1/2 of cell lines being considered were similar just like that in this study and the study of Brenner and Hall[22].
The fraction dose delivery time is another factor that impacts the effect of cell survival. For HepG2 in this study, the differences of surviving fraction in each group irradiated with different prolonged fraction delivery time were small but significant (P<0.05).
These important factors synthetically affect the effect of prolonged fraction delivery time on cell killing. According to the results of this study, HepG2 and Hep3b have similar T1/2. The predominant factor that affects the effect of prolonged fraction delivery time on cell killing apparently should be the SLDR capability of the cells.
In conclusion, the prolonged fraction delivery time modeling IMRT significantly decreased the cell killing in HepG2 but not in Hep3b. The capability of SLDR was the predominant factor determining the cell killing decrease. These effects, if confirmed by clinical studies, should be considered in designing IMRT treatments.
Edited by Li WZ Language Editor Elsevier HK
| 1. | Bortfeld TR, Kahler DL, Waldron TJ, Boyer AL. X-ray field compensation with multileaf collimators. Int J Radiat Oncol Biol Phys. 1994;28:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 313] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Brahme A. Recent advances in light ion radiation therapy. Int J Radiat Oncol Biol Phys. 2004;58:603-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Siochi RA. Minimizing static intensity modulation delivery time using an intensity solid paradigm. Int J Radiat Oncol Biol Phys. 1999;43:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Webb S. The physical basis of IMRT and inverse planning. Br J Radiol. 2003;76:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Wu DH, Liu L, Chen LH. Therapeutic effects and prognostic factors in three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2004;10:2184-2189. [PubMed] |
| 6. | Galvin JM, Chen XG, Smith RM. Combining multileaf fields to modulate fluence distributions. Int J Radiat Oncol Biol Phys. 1993;27:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Wang JZ, Li XA, D'Souza WD, Stewart RD. Impact of prolonged fraction delivery times on tumor control: a note of caution for intensity-modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys. 2003;57:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Thames HD. An 'incomplete-repair' model for survival after fractionated and continuous irradiations. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;47:319-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 260] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Elkind MM. The initial part of the survival curve: does it predict the outcome of fractionated radiotherapy? Radiat Res. 1988;114:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Elkind MM, Sutton HG. X-ray damage and recovery in mammalian cells in culture. Nature. 1959;184:1293-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 303] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Boothman DA, Bouvard I, Hughes EN. Identification and characterization of X-ray-induced proteins in human cells. Cancer Res. 1989;49:2871-2878. [PubMed] |
| 12. | Carney DN, Mitchell JB, Kinsella TJ. In vitro radiation and chemotherapy sensitivity of established cell lines of human small cell lung cancer and its large cell morphological variants. Cancer Res. 1983;43:2806-2811. [PubMed] |
| 13. | Kelland LR, Bingle L, Edwards S, Steel GG. High intrinsic radiosensitivity of a newly established and characterised human embryonal rhabdomyosarcoma cell line. Br J Cancer. 1989;59:160-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Weichselbaum RR, Beckett MA, Vijayakumar S, Simon MA, Awan AM, Nachman J, Panje WR, Goldman ME, Tybor AG, Moran WJ. Radiobiological characterization of head and neck and sarcoma cells derived from patients prior to radiotherapy. Int J Radiat Oncol Biol Phys. 1990;19:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Weichselbaum RR, Rotmensch J, Ahmed-Swan S, Beckett MA. Radiobiological characterization of 53 human tumor cell lines. Int J Radiat Biol. 1989;56:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Barranco SC, Romsdahl MM, Humphrey RM. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971;31:830-833. [PubMed] |
| 17. | Wang JZ, Guerrero M, Li XA. How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;55:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 741] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 521] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 521] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 21. | Steel GG, Deacon JM, Duchesne GM, Horwich A, Kelland LR, Peacock JH. The dose-rate effect in human tumour cells. Radiother Oncol. 1987;9:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 197] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Brenner DJ, Hall EJ. Conditions for the equivalence of continuous to pulsed low dose rate brachytherapy. Int J Radiat Oncol Biol Phys. 1991;20:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 224] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Kampinga HH, Hiemstra YS, Konings AW, Dikomey E. Correlation between slowly repairable double-strand breaks and thermal radiosensitization in the human HeLa S3 cell line. Int J Radiat Biol. 1997;72:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ang KK, Jiang GL, Guttenberger R, Thames HD, Stephens LC, Smith CD, Feng Y. Impact of spinal cord repair kinetics on the practice of altered fractionation schedules. Radiother Oncol. 1992;25:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Millar WT, Canney PA. Derivation and application of equations describing the effects of fractionated protracted irradiation, based on multiple and incomplete repair processes. Part 2. Analysis of mouse lung data. Int J Radiat Biol. 1993;64:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | van Rongen E, Thames HD, Travis EL. Recovery from radiation damage in mouse lung: interpretation in terms of two rates of repair. Radiat Res. 1993;133:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Stewart RD. Two-lesion kinetic model of double-strand break rejoining and cell killing. Radiat Res. 2001;156:365-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Guerrero M, Stewart RD, Wang JZ, Li XA. Equivalence of the linear-quadratic and two-lesion kinetic models. Phys Med Biol. 2002;47:3197-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |