Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1227
Revised: October 4, 2003
Accepted: October 7, 2003
Published online: April 15, 2004
AIM: The pathophysiology underlying gastrointestinal complications of long-standing diabetes is poorly understood. Recent evidence suggests an important role of intestitial cells of cajal in controlling gastrointestinal motility. The aim of this study was to clarify the changes of ultrastructural characteristics of interstitial cells of cajal in stomach of diabetic gastro-electric dysrhythmic rats.
METHODS: Rats were randomly divided into diabetic group and control group, the model of diabetic rats was established by peritoneally injection of streptozotocin. Electrogastrograms were recorded and intestitial cells of cajal in antrum were observed by electrictelescopy after diabetic model rat was established for 3 mo.
RESULTS: In the rats of diabetic group, the gastro-electric dysrhythmia was increased compared with control group, the abnormal rhythm index and the cofficient of variation of slow wave frequency were significantly higher than those of normal rats. The number of the gap junctions of interstitial cells of cajal in antrum of diabetic rats was significantly decreased, and the remaining structures were damaged. The organelles were also damaged, and vacuoles were formed.
CONCLUSION: It is possible that changes in ultrastructural characteristics of interstitial cells of cajal in stomach are one of the mechanisms underlying gastro-electric dysrhythm in diabetic rats.
- Citation: Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J Gastroenterol 2004; 10(8): 1227-1230
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1227.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1227
Gastric motility abnormity occurs in up to 30-50% of patients with long-standing diabetes. Symptoms of diabetic gastropathy can range from mild dyspepsia to recurrent vomiting and abdominal pain and may progress to irreversible end-stage gastric failure known as gastroparesis. Gastroparesis seriously affects quality of life. There is deterioration in glycemic control and incapacitating symptoms such as malnutrition, water and electrolyte imbalance, and aspiration may occur. However, the pathophysiology of diabetic gastropathy and gastroparesis, including impaired fundic and pyloric relaxation and impaired electrical pacemaking, is still not delineated[1-2]. It is generally considered that diabetic gastropathy and gastroparesis may be due to visceral autonomic neuropathy, hyperglucose and smooth muscle degeneration.
Interstitial cells of cajal (ICCs) are a unique class of cells dispersed in gastrointestinal tracts of mammals. In the region of gastric corpus and antrum, multipolar interstitial cells of cajal form two dimensional networks, and have been mistaken for neurons, glial cells, smooth muscle cells, macrophages and fibroblasts. In fact they are mesenchymal in origin. As interstitial cells of cajal develop, they assume major gastrointestinal motility. Interstitial cells of cajal are the pacemaker cells responsible both for initating slow wave activity in gastrointestinal muscles and for active propagation of electrical slow wave. These cells also mediate motor inputs from the enteric nervous system.
Studies have also suggested that many gastrointestinal motor disorders have changes of number and/or structure of interstitial cells of cajal[3-9]. In this study, we established the model of diabetic rats by peritoneal injection of streptozotocin, and intended to investigate the reason of gastric-electrical dysrhythmias by recording the gastric electrical activity and characterizing the ultrastructural features of interstitial cells of cajal in the antrum.
Fifty 2-mo-old healthy Wistar rats (weighing 100-160 g) of either sex were obtained from Animal Center of Third Military Medical Univisity and were divided randomly into control group (n = 20) and diabetic group (n = 30). After fasted overnight, diabetic group rats were injected intraperitoneally with streptozotocin (60 mg/kg) and control group rats were injected intraperitoneally with saline. Blood glucose concentration, weight, appetite and urine volume were measureed every two weeks. In this test, diabetic group animals were considered as diabetic if blood glucose levels exceeded 16.9 mmol/L and were eliminated if blood glucose levels remained < 16.9 mmol/L.
Gastric-electrical activities of control and diabetic group rats were recorded afte 3 mo. After fasted over 12 h, rats were operated under anaesthesia with 3% soluble pentobarbitone (30 mg/kg) intraperitoneally. A pair of stainless platinum wires was implanted in deep muscular plexus of antrum. The wires were arranged in an arching line along the greater curvature about 0.5 cm apart from pylorus. The distance between electrodes was about 0.3 cm apart. The electrodes were affixed to the serosa by nonabsorbable sutures in the seromuscular layer of stomach. Teflon-insulated wires were brought out from nape through the anterior abdominal wall percutaneously. After surgery, the rats were transferred to a recovery cage for a week. After fasted overnight, rats underwent anaesthesia with 3% soluble pentobarbitone (30 mg/kg) intraperitoneally. Wires were connected with a gastro-electrical activity amplifier, and recorded parameters were adjusted. All recorded signals were displayed and simultaneously recorded on computer. Gastric-electrical activity recorded lasting for 60 min. All recorded signals were analyzed by computer. In this study, the frequency of normal rat gastric slow wave was 4.41 ± 0.91/min and its normal range was 2.63-6.19/min. Gastric slow wave was defined as bradygastria if its frequency was lower than 2.63/min and had a duration of longer than 1 min and form of slow wave was ruled and rhythm was in good order. Gastric slow wave was defined as tachygastria if its freguency was greater than 6.19/min and had a duration of longer than 1 min and form of slow wave was ruled and rhythm was in good order. Gastric slow wave was defined as dysrhythmias if its form of slow wave was not ruled and rhythm was in bad order and had a duration of longer than 1 min.
After gastric–electrical activity was recorded, the abdomen was opened immediately and antrum was removed (antrum was cut apart 0.5 cm from pylorus) and fixed containing 3% glutaraldehyde and 4% paraformaldehyde. The specimens were rinsed twice in 0.2 mol/L saccharum-phosphate buffer and post-fixed in 10 g/L osmium tetroxide for 2 h at 4 °C. The specimens were then rinsed in distilled water, block-stained with saturated uranyl acetate solution for 3 h, dehydrated in a grade series of acetone and embedded in epoxy resin 618. Ultrathin sections were cut and double-stained with uranyl acetate and lead citromalic for observation under JEOL-2000EX electron microscope.
Values in the text were expressed as mean ± SE. Statistically significant differences were tested using an analysis of variance. A P value < 0.05 was taken as statistically significant.
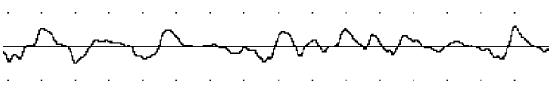
Slowform of normal rats was regular (Figure 1), the mean frequency of control rats was 4.41 ± 0.91 cpm (range: 2.63-6.19 cpm), the abnormal rhythm index was 18.00 ± 4.96% and the cofficient of variation of slow wave frequency was 10.00 ± 6.46%. Compared with normal rats, diabetic rats had less chance of normal rhythm, the mean frequency was 4.03 ± 1.23 cpm (range: 1.62-6.44 cpm), its slowform was irregular (Figure 2). The abnormal rhythm index was 30.62 ± 7.38% and the cofficient of variation of slow wave frequency was 23.50 ± 2.98%. Both of them were significantly higher than those of normal rats (P < 0.01).
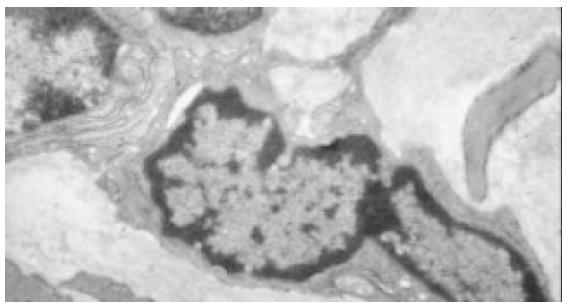
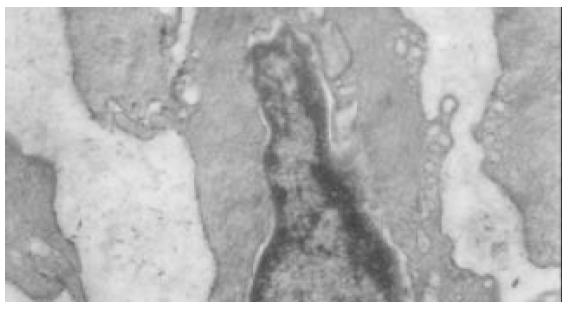
In the normal rat antrum (Figure 3), interstitial cells of cajal were located within the circular muscle layer (ICC-CM) and in the myenteric region (ICC-AP) and were in closely associated with small nerve bundles. These cells were interconnected with each other and neighboring smooth muscle cells via a number of large gap junctions. Interstitial cells of cajal in rat stomach were characterized by abundant mitochondria. Numerous caveolae were observed on cell membranes. The nucle were big and irregular, heterochromatins were often observed on the nuclear membrane and its strucure was clear. Their cytoplasm was usually less, and one or more processes were observed. Intermediate filaments, golgi apparatus, rough (RER) and smooth (RER) endoplasmic reticulum were abundant. Variation in the ultrastructural features of the intestitial cells of cajal in different regions was also observed. ICC-CM was characterized by dense cytoplasm, no basal lamina could be clearly identified. ICC-AP was characterized by numerous caveolae and a distinct basal lamina. In the antrum of diabetic rats (Figure 4), interstitial cells of cajal were fewer. The number of the gap junctions between interstitial cells of cajal and neuron cells, between interstitial cells of cajal and smooth muscle cells, and between themselves were decreased significantly. The remaining structures of those gap junctions were also damaged. Basal lamina was discontinuous and not distinct, some were apart from cell membrane and formed cavum. The organelles-mitochondria and ribosome, for example, were also significantly decreased. Mitochondria were swollen, vacuoles and cytoplasm were dissolved, vacuole and myelin figures were formed. Endoplasmic reticulum was dilated, cytoplasm was dissolving, vacuoles were formed and distributed along plasma membrane, perinuclear space was broadened.
Despite reports of diabetic gastroparesis without autonomic neuropath, this disease, and, particularly, impaired electrical pacemaking, are usually considered to be the result of systemic and/or enteric neuropathies. Recent studies in spontaneously diabetic BioBreeding/Worcester rats and in streptozotocin-diabetic rats have demonstrated decreased nitric oxide synthase expression and reduced nitrergic motor inputs to the stomach, as well as impaired intracellular signaling in response to excitatory neurotransmitters in gastric smooth muscle. Clearly, these defects may contribute to the many abnormal features of diabetic gastropathy. However, it is unclear how impaired neural inputs (especially impaired inhibitory inputs) or impaired smooth muscle response to cholinergic stimulation could result in the loss of slow-wave activity, which has been observed in both humans and animals with diabetes.
Interstitial cells of Cajal were originally described in the gut more than hundred years ago by Ramón y cajal[5]. He characterized “interstitinal neurons” as “primitive accessory components that perhaps modify smooth muscle contraction, subject themselves to regulation from principal neurones”. Cajal provided us with detailed pictures of methylene blue-stained networks of interstitial cells of cajal, which were described as spindle shaped or stellate cells with long, ramified cell processes and large, oval, nuclei with sparse perinuclear cytoplasm, and intercalated between autonomic nerve endings and smooth muscle cells. Interstitial cells of cajal were found within discrete locations within the tunica muscularis throught the gastrointestinal tract and classified into five types according to different locations, i.e., ICC-AP (Auerbach’s myenteric plexus) located between the circular and longitudinal muscle layers, ICC-DMP (deep muscular plexus) located between the inner thin and outer thick sublayers of the circular smooth muscle, ICC-SMP (submuscular plexus) located at the submucosal border, ICC-CM located within the outer thick circular muscle layer; and ICC-LM located within the longitudinal muscle layer.
Many studies have indicated that each organ manifests a unique pattern of distribution of interstitial cells of cajal. In the stomach of rats, we observed interstitial cells of cajal located in close association with small nerve bundles within the circular muscle layer (ICC-CM) and in the myenteric region (ICC-AP), no interstitial cells of cajal were found at the most inner region of the circular muscle layer, submucosal border and deep muscular plexus.
Though there are a large number of methods for indentification of interstitial cells of cajal, such as methylene blue staining, zinc-iodide osmic acid (ZIO)-staining and immunohistochemistry using antibodies against kit proteink. The “gold-standard” for indentification of interstitial cells of cajal is still a combination of structural features in transmission electron microscopy. Although some structural varations have been described to be between species and between various regions of the gastrointestinal tract, the Interstitial cells of cajal ultrastructure are characterized by a combination of the following features: numerouly large and often elongated mitochrondrial profiles, large bundles of intermediate filaments, absence of thick filaments, presence of surface caveoli variably developed basal lamina, synapse-liked contacts between Interstitial cells of cajal and tertiary nerve bundles, well-developed smooth endoplasmic reticulum and often also rough endoplasmic reticulum, close apposition or gap junction contact with smooth muscle cells.
Interstitial cells of cajal have ultrastructural distinction from fibroblasts or smooth muscle cells. Some interstitial cells of cajal may have muscle-like ultrastructural features, such as a basal lamina, caveolae, subsurface cistern and gap junctions. For this reason, some interstitial cells of cajal have been considered as modified or specialized smooth muscle cells. Howerer, the interstitial cells of cajal do not contain the well-organized contractile apparatus characteristic of muscle cells. Some interstitial cells of cajal may have an appearance similar to fibroblasts and lack clear muscle-like features. However, even in these cases, they are distinguishable from fibroblast-like cells by a combination of features including a characteristic electron density of cytoplasm, large gap junctions, abundant intermediate filaments, numerous mitochondria, well-developed SER and flattened cisterns of RER. Furthermore, a great number of collagen fibers often distribute around fibrobalst cells. Morphological observations have led to a number of hypotheses on the possible physiological roles of interstitial cells of cajal[10-18]. (1) These cells may be the source of slow electrical waves in gastrointestinal tract. (2) They participate in conduction of electrical currents, and (3) They mediate neural signals between enteric nerves and muscles.These hypotheses have been tested by experiments. (1) Slow electrical waves in gastrointestinal muscle strips were absent when interstitial cells of cajal were removed by dissection or lesioned by cytotoxic chemicals. (2) Electrophysiological experiments on isolate cells confirmed that interstitial cells of cajal could generate rhythmic electrical activity and also respond to messenger molecules known to be released from enteric nerves. (3) In Ws/Ws mutant rats, or in mice treated with antibody against the protein c-kit, slow wave activity was impaired. (4) Slow wave passively decayed as a function of distance from the pacemaker appeared. (5) After removal of interstitial cells of cajal, smooth muscle cells continued to be excitable, but in the absence of interstitial cells of cajal, smooth muscle produced action potentials rather than slow wave-like activity. Studies have also suggested that many diseases of gastrointestinal motor disorders have changes in number and/or structure of Interstitial cells of cajal[3-9]. In infants with hypertrophic pyloric stenosis, there was a significant decrease in the number of interstitial cells of cajal, this decrease was prominent in the ICC-AP. In some patients with pseudo-obstruction, there was a marked decrase in the number of interstitial cells of cajal. Constipation was a very prevalent motility problem, but its underlying mechanisms were obscure. Studies found that the volume of interstitial cells of cajal in the colon of patients with slow transit constipation was significantly decreased compared with normal controls.
Normal gastric emptying requires the proper function of the gastric electrical pacemaker system. Gastroparesis has been associated with electrical abnormalities, and deviations from normal slow-wave rhythm (dysrhythmias) have been reported to result in delayed gastric emptying. In this study, we established the model of diabetic rats by peritoneally injection of streptozotocin, and recorded the gastro-electrical activity after 3 mo. We discovered that in the rats of diabetic group, the gastro-electric dysrhythmia was increased compared with control group,the number of interstitial cells of cajal in antrum of diabetic rats was significantly decreased and the number of the gap junctions of interstitial cells of cajal also was significantly decreased, and the remaining structures were damaged. The organelles-mitochondria and ribosome, for example, were also significantly decreased. Mitochondria were swollen, myelin figures were formed. Endoplasmic reticulum was dilated, cytoplasm was dissolved, vacuoles were formed and distributed along plasma membrane, perinuclear space broadened. This shows that degeneration of interstitial cells of cajal is responsible for gastro-electrical dysrhythmias of diabetic rats, the identification of abnormalities in interstitial cells of cajal in diabetic gastro-electric dysrhythm offers a potential future therapeurapy.
Edited by Hu DK and Wang XL Proofread by Xu FM
| 1. | Qi HB, Luo JY, Dai XG, Wang XQ. A study on motility in patients with diabetic gastroparesis. Clin J New Gastroenterol. 1999;5:661-662. |
| 2. | Quigley EM. The evaluation of gastrointestinal function in diabetic patients. World J Gastroenterol. 1999;5:277-282. [PubMed] |
| 3. | Long QL, Fang DC. Function of interstitial cells of cajal in gastrointestinal tract. Shijie Huaren Xiaohua Zazhi. 2002;10:352-355. |
| 4. | Huizinga JD, Berezin I, Sircar K, Hewlett B, Donnelly G, Bercik P, Ross C, Algoufi T, Fitzgerald P, Der T. Development of interstitial cells of Cajal in a full-term infant without an enteric nervous system. Gastroenterology. 2001;120:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Der T, Bercik P, Donnelly G, Jackson T, Berezin I, Collins SM, Huizinga JD. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology. 2000;119:1590-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 7. | He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Hudson N, Mayhew I, Pearson G. A reduction in interstitial cells of Cajal in horses with equine dysautonomia (grass sickness). Auton Neurosci. 2001;92:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Sandgren K, Larsson LT, Ekblad E. Widespread changes in neurotransmitter expression and number of enteric neurons and interstitial cells of Cajal in lethal spotted mice: an explanation for persisting dysmotility after operation for Hirschsprung's disease? Dig Dis Sci. 2002;47:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Koh SD, Kim TW, Jun JY, Glasgow NJ, Ward SM, Sanders KM. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol. 2000;527 Pt 1:149-162. [PubMed] |
| 12. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Dickens EJ, Edwards FR, Hirst GD. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol. 2001;531:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Daniel EE, Thomas J, Ramnarain M, Bowes TJ, Jury J. Do gap junctions couple interstitial cells of Cajal pacing and neurotransmission to gastrointestinal smooth muscle? Neurogastroenterol Motil. 2001;13:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Salmhofer H, Neuhuber WL, Ruth P, Huber A, Russwurm M, Allescher HD. Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell Tissue Res. 2001;305:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003;311:299-313. [PubMed] |
| 17. | Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |