Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1198
Revised: December 14, 2003
Accepted: December 16, 2003
Published online: April 15, 2004
AIM: Hepatic ischemia/reperfusion injury may cause acute inflammatory, significant organ damage or dysfunction, and remains an important problem for liver transplantation. Our previous in vivo and in vitro studies demonstrated that Yisheng injection (YS), a traditional Chinese medicine, had protective effect on ischemia/reperfusion injury. In this study, we examined whether YS had protective effect for hepatic ischemia/reperfusion injury and explored its protective mechanism.
METHODS: Hepatic warm ischemia/reperfusion was induced in mice. YS at different doses (5, 10, 20 mg/kg) was injected intraperitoneally 24 h and 1 h before ischemia and a third dose was injected intravenously just before reperfusion. The hepatocellular injury, oxidative stress, neutrophil recruitment, proinflammatory mediators and adhesion molecules associated with hepatic ischemia/ reperfusion injury were assayed by enzyme-linked immunosorbent assay (ELISA), immunohistochemical assay and reverse transcription polymerase chain reaction (RT-PCR).
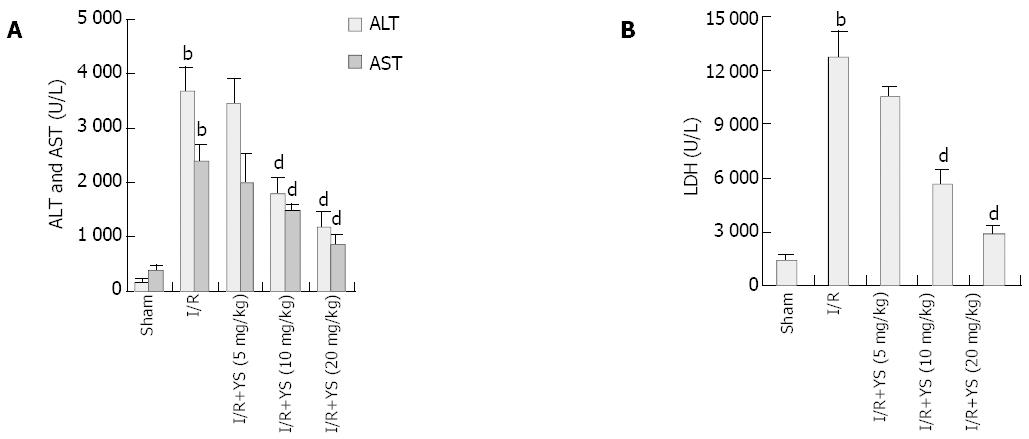
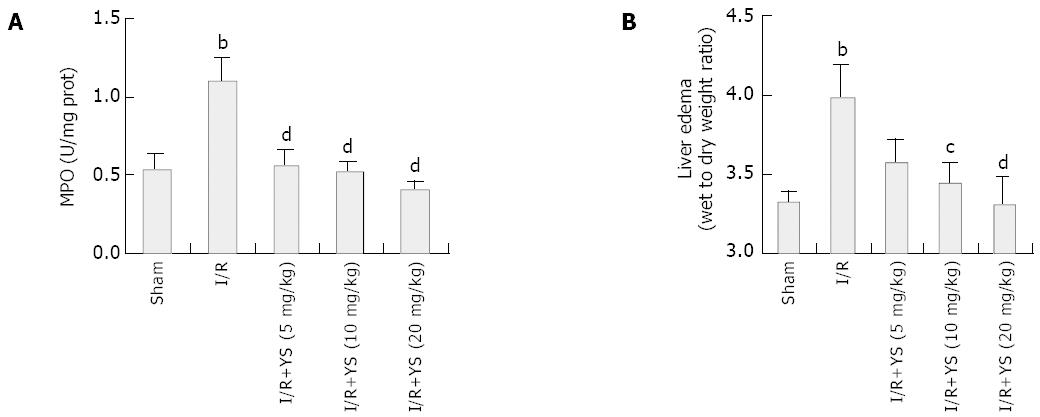
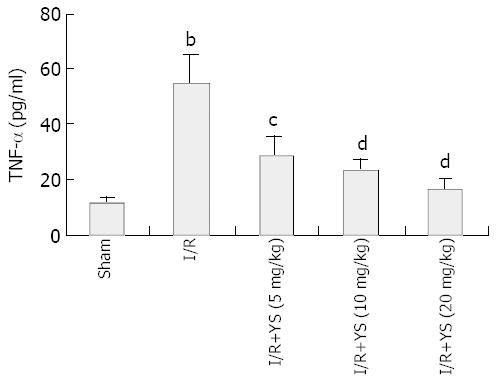
RESULTS: Undergoing 90 min of ischemia and 6 h of reperfusion caused dramatical injuries in mouse livers. Administration of YS at doses of 5, 10 and 20 mg/kg effectively reduced serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH), from 3 670 ± 463 U/L, 2 362 ± 323 U/L and 12 752 ± 1 455 U/L in I/R group to 1 172 ± 257 U/L, 845 ± 193 U/L and 2 866 ± 427 U/L in YS (20 mg/kg) treated group, respectively (P < 0.01). The liver myeloperoxidase (MPO) and malondialdehyde (MDA) contents were decreased from 1.1 ± 0.2 (U/mg protein) and 9.1 ± 0.7 (nmol/mg protein) in I/R group to 0.4 ± 0.1 (U/mg protein) and 5.5 ± 0.9 (nmol/mg protein) in YS (20 mg/kg) treated group, respectively (P < 0.01). Moreover, the serum levels of tumor necrosis factor-alpha (TNF-α) were reduced from 55 ± 9.9 (pg/mL) in I/R group to 16 ± 4.2 (pg/mL) (P < 0.01). Furthermore, the over-expressions of TNF-α and intercellular adhesion molecule-1 (ICAM-1) were suppressed by YS treatment in a dose-dependent manner.
CONCLUSION: YS attenuates hepatic warm ischemia/reperfusion injury by reducing oxidative stress and suppressing the over-expression of proinflammatory mediators and adhesion molecules.
- Citation: Cheng F, Li YP, Cheng JQ, Feng L, Li SF. The protective mechanism of Yisheng Injection against hepatic ischemia reperfusion injury in mice. World J Gastroenterol 2004; 10(8): 1198-1203
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1198.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1198
Clinical and experimental evidences suggest that an initial insult to organ allografts may influence both early and late functional survival. This injury may be either immunologic or antigen independent[1].
Hepatic ischemia/reperfusion injury is an important nonimmunologic factor and remains a significant problem and limitation of liver transplantation and may result in liver failure, remote organ failure, and even death[2,3].
During the initial phase of hepatic ischemia-reperfusion injury, Kupffer cells are activated and release reactive oxygen species (ROS)[4] and proinflammatory cytokines[5], especially tumor necrosis factor-alpha (TNF-α)[6,7]. The enhanced production of TNF-α plays an important role in the initiation of a cascade of events that causes significant liver injury mediated by neutrophils. One of the main functions of TNF-α is to up-regulate the expression of adhesion molecules, especially intercellular adhesion molecule-1 (ICAM-1), and then mediate the recruitment of neutrophils into the liver resulting in further hepatic injury[8,9].
Ischemia-reperfusion injury becomes significant through rapid up-regulation and surface expression of adhesion molecules, and activation of circulating host leukocytes and their binding to the vascular endothelium[10]. In addition, the insult from ischemia/reperfusion surrounding engraftment may trigger alloresponsiveness in the host, making it potentially more prone to rejection[11]. Furthermore, the initial acute rejection injury predisposes to chronic graft dysfunction early injuries may also affect later events. For this reason, the protection of liver against ischemia-reperfusion injury (IRI) remains one of the major nonimmunologic problems of liver transplantation[12].
Our previous studies demonstrate that Yisheng injection (YS), a herbal preparation developed from traditional Chinese medicine, can effectively protect endothelial cells against hypoxia/reoxygenation injury[13,14]; decrease the blood viscosity in hypostasis rats[15]; attenuate the sclerosis of transplanted abdominal aorta in rats by reducing the generation of free radicals and down-regulating the expression of TGF-β[16]; protect isolated testes against cold preservation/reperfusion injury through reducing lipid peroxidation, calcium supercharge and damage to mitochondrial function[17]; and protect kidney against ischemia/reperfusion injury in pigs by reducing serum levels of TNF-α (unpublished results). In this study, we examined whether YS had protective effect for hepatic ischemia/reperfusion injury and explored its protective mechanism.
Yisheng injection (YS, 20 mg/mL) was provided by Chengdu Huayi Drug Co (Chengdu, China). Its effective ingredients are alkaloids extracted from Corydalis thalictrifolia Franch. and Schnabelia oligophylla Hand.-Mazz. In this study, YS was diluted with sterile saline.
Male C57BL/6 mice weighing 22 to 25 g were purchased from Vital River Co, Beijing, China. All mice were provided with standard laboratory chow and water and housed in accordance with institutional animal care policies.
The mice were randomly assigned into five experimental groups as follows: (1) sham operation group, (2) I/R group (treated with saline), (3) small-dose (5 mg/kg of YS) treated group, (4) middle-dose (10 mg/kg of YS) treated group, (5) large-dose (20 mg/kg of YS) treated group.
The model of partial hepatic ischemia and reperfusion was performed with published method[18]. Briefly, 24 h and 1 h before the induction of ischemia, the mice received intraperitoneally two times either sterile saline or YS (5, 10 or 20 mg/kg). They were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally), then a midline laparotomy was performed and an atraumatic clip was used to interrupt the arterial and the portal venous blood supply to the left lob of the liver. After 90 min of partial hepatic ischemia, the mice again received either sterile saline or YS (5, 10 or 20 mg/kg) via the lateral tail vein, and the clip was removed initiating hepatic reperfusion. Sham control mice underwent the same protocol, but without vascular occlusion. Abdomen was sutured by two layers with 4.0 suture.
In order to determine the proper reperfusion time point, we assayed the alanine transaminase (ALT) levels in the blood samples obtained at different time points of 2, 4, 6, 10 and 24 h of reperfusion following 90 min of ischemia, and found that the blood ALT level reached the peak at the end of 6 h of reperfusion (data not shown). So the time point of 6 h of reperfusion was chosen in this study. Mice were killed after 6 h of reperfusion following 90 min of ischemia, and liver tissue (from the ischemic lob) and blood samples were taken for analysis.
Blood was obtained at the time of sacrifice. The plasma concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured in a clinical laboratory as markers of hepatic damage. The serum levels of TNF-α were determined by using an ELISA Kit (Jingmei Biotech Co, Shengzheng, China) according to the maneufactors’ instructions.
The extent of liver edema was measured by tissue wet-to-dry weight ratios. After dissection, liver samples were weighed and then placed in a drying oven at 55 °C until a constant weight was obtained[18]. In this determination, liver edema was represented by an increase in the wet-to-dry weight ratios.
Liver samples were homogenized in 100 mmol/L Tris-HCl buffer and centrifuged at 10 000 g for 20 min, and then liver myeloperoxidase (MPO), superoxide dismutase (SOD) and malondialdehyde (MDA) contents were assayed by using assay kits (Jiancheng Biotech Ltd, Nanjing, China), and the protein contents were assayed by using assay kits (Bio-Rad, United States) according to the maneufactors’ instructions.
Tissue samples were fixed in 40 mg/L buffered formaldehyde and embedded in paraffin. For immunohistologic analysis, 5 μm sections of paraffin-embedded liver tissues were cut, and stained with primary anti-mouse mAbs against TNF-α (diluted, 1:500, Santa Cruz Biotech Co, Santa Cruz, CA) or ICAM-1 (diluted, 1:400, Santa Cruz Biotech Co, Santa Cruz, CA). After incubation, the sections were incubated with peroxidase-conjugated goat anti-mouse IgG. The bound peroxidase was detected using 3,3’-diaminobenzidine.
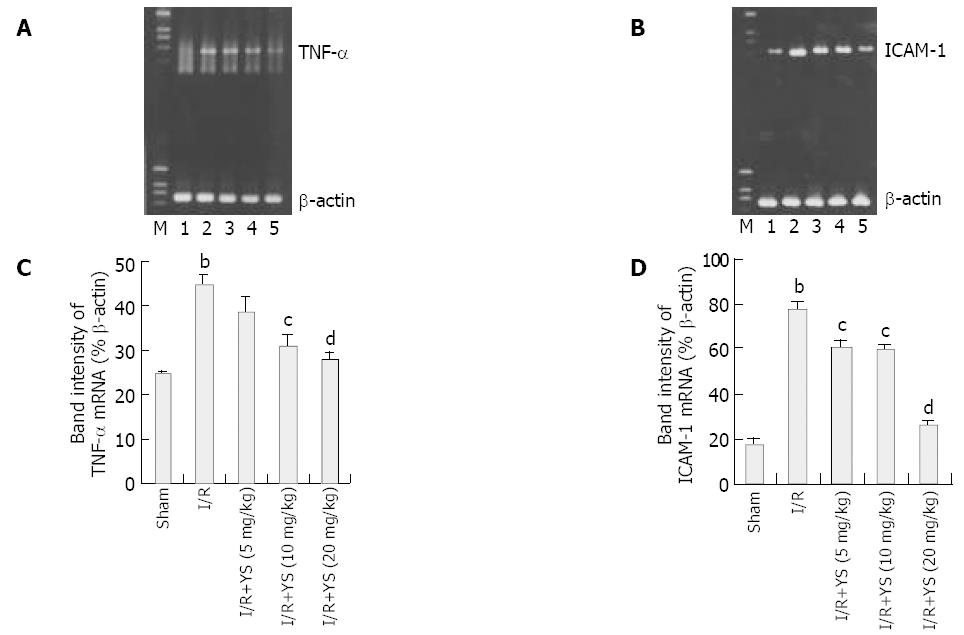
Total RNA was extracted from liver tissue using SV Total RNA Isolation System (Promega Co, Madison, WI, United States), according to the manufacturer’s instructions, and was quantified by UV spectrophotometer. RNA (1 μg) was reverse transcribed and amplified using TaKaRa One-Step RT-PCR Kit (TaKaRa Biotech Co, Dalian, China) at following RT-PCR conditions: 50 °C for 30 min; 30 cycles at 95 °C for 1 min, 59 °C for 90 s, and 72 °C for 2 min. Primers used in PCR reactions were as follows[18]: TNF-α sense (5’-AGCCCACGTAGCAAACCACCAA-3’) and antisense (5’-ACACCCATTCCCTTCACAGAGCAAT-3’), to give a 446-bp product; ICAM-1 sense (5’-TGGAACTGCA CGTGCTGTAT-3’) and antisense (5’-ACCATTCTGTTC AAAAGCAG-3’), to give a 513-bp product; and β-actin sense (5’-CTGAAGTACCCCATTGAACATGGC-3’) and antisense (5’-CAGAGCAGTAATCTCCTTCTGCAT-3’), to give a 672-bp product. PCR products were stained with SYBR green dye, electrophoresed in a 20 g/L agarose gel, and photographed. Digital photographs were assessed with image-analysis software (Image-Pro Plus System, Media Cybernetics Co, United States) and mRNA expressions were evaluated by the band intensity ratios of TNF-α and ICAM-1 to β-actin and presented as percentage of β-actin.
All data were expressed as mean ± SD. Statistical analysis was performed by analysis of variance (ANOVA), and Student’s t test was used to compare individual group means. P values less than 0.05 were considered statistically significant.
Hepatic injury was assessed by measuring serum levels of ALT, AST and LDH. Undergoing 90 min of hepatic ischemia and 6 h of reperfusion caused dramatical increases in serum ALT levels compared with sham controls, from 157 ± 60 U/L to 3670 ± 463 U/L (Figure 1A). Administration of YS at a dose of either 5, 10 or 20 mg/kg reduced the serum ALT levels by about 6.6%, 51.6% and 68.1% respectively, and YS at middle and large doses (10 and 20 mg/kg) showed significant effects (Figure 1A). Measurement of AST and LDH values reflected similar differences among the groups (Figure 1A, B), further demonstrating the dose-dependent protective effects of YS.
Hepatic neutrophil recruitment was determined by liver MPO content and liver edema was determined by tissue wet-to-dry weight ratio. Hepatic ischemia and 6 h of reperfusion caused marked increases in liver MPO content compared with sham controls (Figure 2A). In the presence of YS (5, 10, 20 mg/kg), liver MPO content was significantly reduced by about 49.1%, 52.7% and 63.6% respectively (Figure 2A). In addition, administration of YS also reduced the liver edema in a dose-dependent manner (Figure 2B).
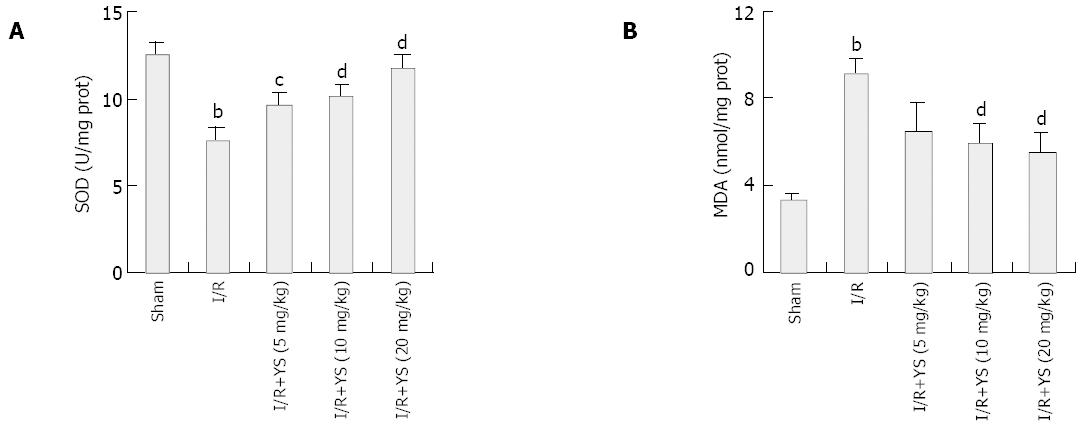
Hepatic ischemia and 6 h of reperfusion caused marked decrease in liver SOD activity (Figure3A) and resulted in remarkable increases in liver MDA content compared with sham controls (Figure 3B). In the presence of YS at doses of 5, 10 and 20 mg/kg, the liver SOD activities were elevated by about 26.6%, 33.6% and 54.8% respectively, and liver MDA content reduced by 28.6%, 35.2% and 39.6% respectively, both in a dose-dependent manner.
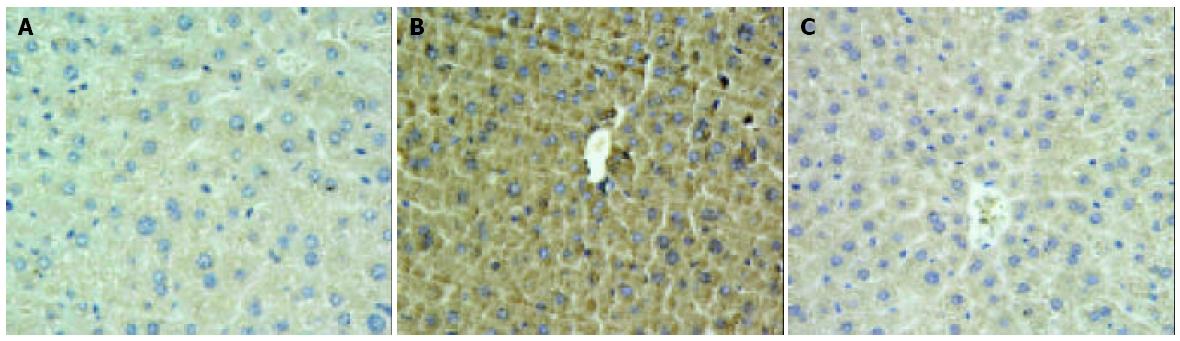
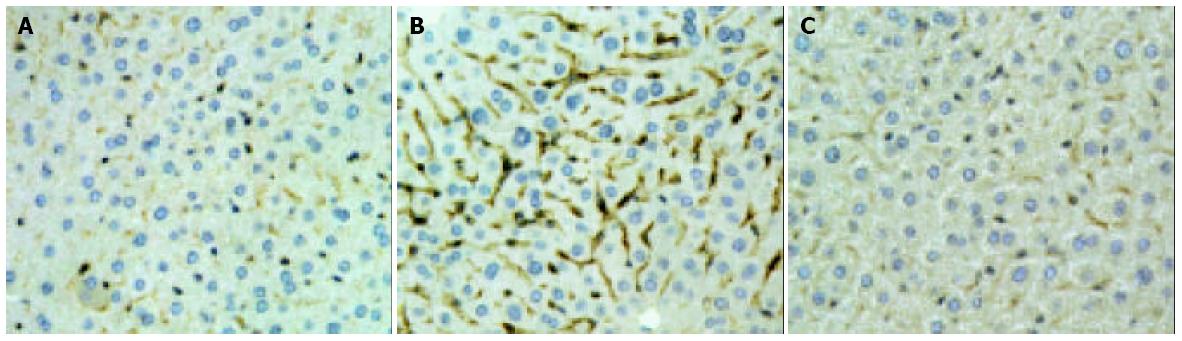
Hepatic ischemia and 6 h of reperfusion markedly increased the levels of TNF-α in sera (Figure 4). Administration of YS at different doses of 5, 10 and 20 mg/kg significantly reduced the serum levels of TNF-α by about 48.2%, 57.3% and 70.9% respectively (Figure 4). The over-expressions of TNF-α and ICAM-1 on liver tissues after I/R were demonstrated by immunohistochemical assay (Figure 5A, B; Figure 6A, B). In the presence of YS (20 mg/kg), hepatic ischemia/reperfusion-induced increases in TNF-alpha and ICAM-1 expression were dramatically suppressed (Figure 5C; Figure 6C).
To determine the expressions of proinflammatory mediators and adhesion molecules, mRNA transcripts for TNF-α and ICAM-1 were assessed. RNA extracts from livers undergoing 90 min of ischemia and 6 h of reperfusion were analyzed by reverse transcription PCR. Furthermore, the band intensity ratios of TNF-α and ICAM-1 to β-actin were evaluated and presented as percentage of β-actin, and were compared among sham, I/R and YS-treated groups. Hepatic ischemia and reperfusion remarkably increased mRNA expression of TNF-α (Figure 7A) and ICAM-1 (Figure 7B), respectively. Administration of YS at doses of 5, 10 and 20 mg/kg significantly abrogated hepatic ischemia/reperfusion-induced increases in TNF-α and ICAM-1 mRNA expression (Figure 7A, B). The comparison of band intensity ratios of TNF-α and ICAM-1 to β-actin in sham, I/R and YS-treated groups demonstrated that YS treatment at doses of 5,10 and 20 mg/kg effectively suppressed the evaluation of TNF-α and ICAM-1 mRNA expression in a dose-dependent manner (Figure 7C, D).
DISCUSSION
Ischemia-reperfusion (I/R) is an unavoidable process in liver transplantation. A major disadvantage of this event is an acute inflammatory response that may cause significant organ damage or dysfunction. Inflammatory organ injury is a primary concern after liver transplantation[18]. Previous studies have identified many of the mediators involved in the pathogenesis of hepatic ischemia/reperfusion injury[4-8]. Among them, ROS, TNF-α, ICAM-1 and neutrophil are central to this process[6,19].
After warm ischemia, ROS produced at the moment of reperfusion activated and promoted the adhesion of leukocytes to microvascular endothelium[20]. Previous studies suggest that free radicals formed during reperfusion are involved in the mechanism of graft failure following liver transplantation in the rat[21]. Moreover, oxidative stress is found to have sustained for a long time after clinical liver transplantation[22], and linked with primary graft nonfunction[23]. Furthermore, studies of lung[24] and renal[25] transplant recipients have suggested that oxidative stress may predispose grafts to acute and chronic rejection. On the other hand, treatment of ROS-scavenging enzymes, such as SOD, can prevent the large increase in MPO activity (an index of tissue neutrophil count) associated with ischemia-reperfusion[26], reduce hepatic oxidative stress, and effectively protect liver against ischemia-reperfusion injury and transplanted liver failure[27,28].
Results in this study showed that treatment of YS at different doses of 5, 10 and 20 mg/kg elevated the liver SOD activities and reduced the hepatic MDA contents in a dose-dependent manner (Figure 3A, B), demonstrating YS’ effects to reduce the oxidative stress caused by hepatic warm ischemia/reperfusion and attenuate subsequent organ damages (Figure 1A,B).
TNF-α produced by activated Kupffer cells is thought to play a central role in the initial phase of ischemia/reperfusion injury[7]. Previous studies have showed that TNF-α caused overexpression of adhesion molecules on both endothelial cells and leukocytes[29]; had multiple effects on neutrophil activity, including increased ROS generation[30]; increased neutrophil aggregation and adhesion to endothelial cells[31]; and resulted in hepatocyte necrosis[32] or apoptosis[33,34]. On the other hand, recent studies have demonstrated that neutralization with specific monoclonal antibodies against TNF-α before ischemia induction can ameliorate apoptosis and attenuate postischemic hepatic injury[35].
Administration of YS significantly reduced the serum levels of TNF-α (Figure 4) and the TNF-α expression on liver tissues (Figure 5), and this effect was confirmed by RT-PCR analysis on TNF-α mRNA in liver tissues (Figure 7A, C). This means YS treatment can effectively reduce the release of TNF-α through suppressing the overexpression of TNF-α mRNA caused by hepatic ischemia/reperfusion, and attenuate subsequent insult events mediated by overexpression of TNF-α, such as up-regulation of adhesion molecules and neutrophil recruitment.
Normally, ICAM-1 is weekly expressed on portal and hepatic endothelium, but in acute rejection and ischemia/reperfusion there is over-expression of ICAM-1 on bile ducts, endothelium, and perivenular hepatocytes[19,20]. ICAM-1 is found to be a potent specific indicator of rejection and may be useful in predicting which patient will progress to irreversible rejection[19].
It has been found that TNF-α can directly up-regulate the expression of ICAM-1 in both lung and liver after hepatic ischemia/reperfusion injury[7], and the over-expression of ICAM-1 may result in the adhesion and transmigration of neutrophils from the vascular lumen into tissue parenchyma[36], ultimately leading to tissue injury and organ dysfunction[18]. Furthermore, blockade of TNF-α decreases hepatic expression of the adhesion molecule ICAM-1 as well as liver neutrophil recruitment[7].
Treatment with YS effectively suppressed the overexpression of ICAM-1 on liver tissue (Figure 6) and abrogated hepatic ischemia/reperfusion-induced increase in ICAM-1 mRNA expression (Figure 7B). As a result, the neutrophil recruitment was effectively reduced by YS treatment (Figure 2A) and the liver edema was attenuated as well (Figure 2B).
In summary, YS treatment significantly reduced the oxidative stress, the overexpression of TNF-α and ICAM-1, and the neutrophil recruitment associated with hepatic ischemia/ reperfusion, and finally effectively protected liver against ischemia/ reperfusion injury. This drug would be a useful tool for preventing liver dysfunction or failure caused by hepatic ischemia/reperfusion injury in liver surgery and liver transplantation.
The authors thank Xia Qing-Jie, Yang Lai-Hong and Zhang Fa-Qiang for assistance with the illustrations.
Edited by Hu DK and Xu FM
| 1. | Tullius SG, Tilney NL. Both alloantigen-dependent and-inde-pendent factors influence chronic allograft rejection. Transplantation. 1995;59:313-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 321] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | González FX, Rimola A, Grande L, Antolin M, Garcia-Valdecasas JC, Fuster J, Lacy AM, Cugat E, Visa J, Rodés J. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology. 1994;20:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. [PubMed] |
| 5. | Jiang Y, Gu XP, Qiu YD, Sun XM, Chen LL, Zhang LH, Ding YT. Ischemic preconditioning decreases C-X-C chemokine expression and neutrophil accumulation early after liver transplantation in rats. World J Gastroenterol. 2003;9:2025-2029. [PubMed] |
| 6. | Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 641] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182-191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368-374. [PubMed] |
| 9. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342-345. [PubMed] |
| 10. | Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Shoskes DA, Parfrey NA, Halloran PF. Increased major histocompatibility complex antigen expression in unilateral ischemic acute tubular necrosis in the mouse. Transplantation. 1990;49:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [PubMed] |
| 13. | Li S, Jiang H, Li Y, Wu D, Zhang L, Zhang L, Cheng J. [A study on the protective mechanism of yisheng injection against the anoxia-reoxygenation injury to endothelial cells]. Hua Xi Yi Ke Da Xue Xue Bao. 2002;33:215-219. [PubMed] |
| 14. | Jiang HM, Li SF, Li YP. [Influence of anoxia/reoxygenation on immunofunction of endothelial cells and effect of intervention with yisheng injection on it]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:451-454. [PubMed] |
| 15. | Yang H, Chen J, Cai S, Li Y, Tang Q, Liu Y. [Effects of YMB injection on hemorheology in rats]. Hua Xi Yi Ke Da Xue Xue Bao. 2000;31:230-232. [PubMed] |
| 16. | Bu H, Chen WG, Liu YB, Wu DR, Cheng JQ, Wang L. Experi-mental study on the protective effect of Yisheng injection on the sclerosis of transplanted abdominal aorta. Huaxi Yaoxue Zazhi. 2000;15:185-188. |
| 17. | Jiang HM, Li M, Li YP. [Experimental study on isolated testes with ischemia/reperfusion injury]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2001;15:377-381. [PubMed] |
| 18. | Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology. 1999;30:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Adams DH, Hubscher SG, Shaw J, Rothlein R, Neuberger JM. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet. 1989;2:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 152] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol. 1989;257:G683-G688. [PubMed] |
| 21. | Henry DC, Wenshi G, Sadayuki N, John JL, Ronald PM, Ronald GT. Evidence that free radicals are involved in graft failure following orthotopic liver transplantation in the rat-an electron paramagnetic resonance spin trapping study. Transplantation. 1992;54:199-204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Burke A, FitzGerald GA, Lucey MR. A prospective analysis of oxidative stress and liver transplantation. Transplantation. 2002;74:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Atalla SL, Toledo-Pereyra LH, MacKenzie GH, Cederna JP. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985;40:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 160] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Shiraishi T, Kuroiwa A, Shirakusa T, Kawahara K, Yoneda S, Kitano K, Okabayashi K, Iwasaki A. Free radical-mediated tissue injury in acute lung allograft rejection and the effect of superoxide dismutase. Ann Thorac Surg. 1997;64:821-825. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Cristol JP, Maggi MF, Vela C, Descomps B, Mourad G. Lipid metabolism and oxidative stress in renal transplantation: implications for chronic rejection. Transplant Proc. 1996;28:2820-2821. [PubMed] |
| 26. | Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567-G574. [PubMed] |
| 27. | Wheeler MD, Katuna M, Smutney OM, Froh M, Dikalova A, Mason RP, Samulski RJ, Thurman RG. Comparison of the effect of adenoviral delivery of three superoxide dismutase genes against hepatic ischemia-reperfusion injury. Hum Gene Ther. 2001;12:2167-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Lehmann TG, Wheeler MD, Froh M, Schwabe RF, Bunzendahl H, Samulski RJ, Lemasters JJ, Brenner DA, Thurman RG. Effects of three superoxide dismutase genes delivered with an adenovirus on graft function after transplantation of fatty livers in the rat. Transplantation. 2003;76:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Issekutz TB. Effects of six different cytokines on lymphocyte adherence to microvascular endothelium and in vivo lympho-cyte migration in the rat. J Immunol. 1990;144:2140-2146. |
| 30. | Tsujimoto M, Yokota S, Vilcek J, Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986;137:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Meyer JD, Yurt RW, Duhaney R, Hesse DG, Tracey KJ, Fong YM, Verma M, Shires GT, Dineen P, Lowry SF. Tumor necrosis factor-enhanced leukotriene B4 generation and chemotaxis in human neutrophils. Arch Surg. 1988;123:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Yu YY, Si CW, Tian XL, He Q, Xue HP. Effect of cytokines on liver necrosis. World J Gastroenterol. 1998;4:311-313. [PubMed] |
| 33. | Zang GQ, Zhou XQ, Yu H, Xie Q, Zhao GM, Wang B, Guo Q, Xiang YQ, Liao D. Effect of hepatocyte apoptosis induced by TNF-α on acute severe hepatitis in mouse models. World J Gastroenterol. 2000;6:688-692. |
| 34. | Streetz K, Leifeld L, Grundmann D, Ramakers J, Eckert K, Spengler U, Brenner D, Manns M, Trautwein C. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology. 2000;119:446-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Ben-Ari Z, Hochhauser E, Burstein I, Papo O, Kaganovsky E, Krasnov T, Vamichkim A, Vidne BA. Role of anti-tumor necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation. 2002;73:1875-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Yadav SS, Howell DN, Gao W, Steeber DA, Harland RC, Clavien PA. L-selectin and ICAM-1 mediate reperfusion injury and neutrophil adhesion in the warm ischemic mouse liver. Am J Physiol. 1998;275:G1341-G1352. [PubMed] |