Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1162
Revised: November 15, 2003
Accepted: December 16, 2003
Published online: April 15, 2004
AIM: The prevention of recurrence of colon cancer (CC) after operation is very important for improvement of the prognosis of CC patients, especially those with micro-metastasis. The generation of fused cells between dendritic cells (DCs) and tumor cells maybe an effective approach for tumor antigen presentation in immunotherapy. In this study, we fused human colon caner SW480 cells and human peripheral blood - derived DCs to induce an antitumor activity against human CC.
METHODS: CC SW480 cells and human peripheral blood - derived DCs were fused with 500 mL/L polyethylene glycol (PEG).
RESULTS: The specific T cell responses activated by fusion cells (FCs), were observed. About 100 mL/L to 160 mL/L of the PEG-treated non-adherent cells with fluorescences were considered to be dendritomas that highly expressed the key molecules for antigen presentation in our five cases. In vitro studies showed that fusions effectively activated CD8+ T lymphocytes to secrete interferon-γ. The early apoptotic ratio of the colon cancer SW480 cells was higher than that of controls, which was affected by cytotoxic T lymphocytes (CTLs) stimulated by dendritomas.
CONCLUSION: The data indicate that fusion of tumor cells with DCs is an attractive strategy to induce tumor rejection.
-
Citation: Xu F, Ye YJ, Wang S.
In vitro antitumor immune response induced by fusion of dendritic cells and colon cancer cells. World J Gastroenterol 2004; 10(8): 1162-1166 - URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1162
Colon cancer (CC) is one of the most common malignancies in the Western world. As diet custom has changed these years, the number of cases is increasing in the Eastern world. Although surgical resection is the first choice wordwide, an effective approach for the treatment of CC patients with metastasis and cancer recurrence postoperation has not yet been found.
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) which are the prime naive T cells and initiate a prime immune response[1,2]. Various DC-based strategies, such as DCs pulsed with tumor-associated peptides or proteins, viral transduction of DCs with tumor-specific genes or transfection with liposomal DNA or RNA, have been developed to introduce tumor specific antigens into DCs and thereby to generate cytotoxic T lymphocyte (CTL) responses against malignant cells[3-9]. However, few tumor-specific antigens have been identified, and their immunogenicity is uncertain in most malignant tumors.
An attractive approach to the enhancement of antitumor activity is to generate the fusions between tumor cells and DCs[10]. Multiple tumor antigens, including those unidentified yet, are processed endogenously and presented to T lymphocytes by the MHC class I and II pathways in the context of costimulatory signals[11]. Some inspiring results have been reported in vitro and in vivo except that of human colon cancer due to the difficulty in isolating the tumor antigens from tumor tissues and the facility contaminated for primary culture[12-16].
In this study, we fused human colon caner SW480 cells and human peripheral blood - derived DCs to induce an antitumor activity against human CC, because they shared some common antigens between cells from tissue and cell line SW480.
Human SW480 colon cancer cells (ATCC#CCL-228) were grown in RPMI medium 1640 supplemented with 100 mL/L heat-inactivated FCS.
Peripheral blood mononuclear cells (PBMC) were isolated from patients with CC by Ficoll-Hypaque density-gradient centrifugation. The pure CD14+ PBMC and CD8+ T lymphocytes were isolated by MACS magnetic microbeads (Miltenyi, Germany) respectively according to the manufacturer’s directions. CD14 PBMC were cultured for 1 wk in RPMI medium 1 640/10% human serum containing 1 000 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 1 000 U/mL IL-4. CD8+ T lymphocytes were cultured in RPMI medium 1 640/10% human serum containing 20 u/mL IL-2.
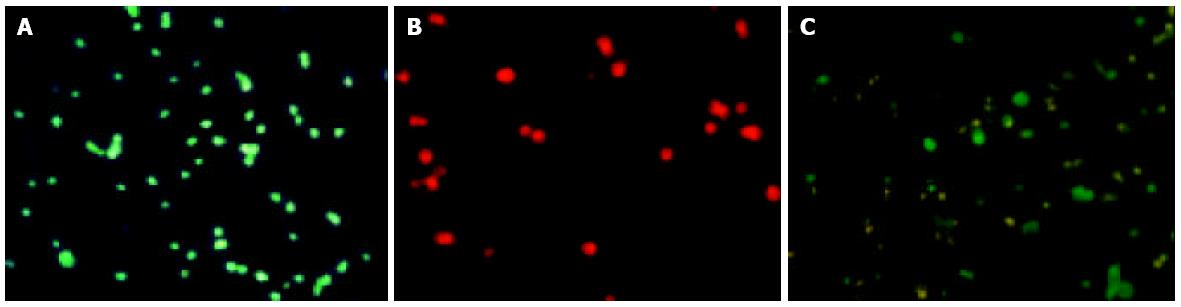
Tumor cells and DCs were stained red and green, respectively, using PKH26-GL and PKH67-GL kits (Sigma) according to the manufacture’s directions. The tumor cells were irradiated at a dose of 30Gy before staining. Human DCs and tumor cells were fused together by mixing the two types at a ratio of 3:1 in a 15-mL conical centrifuge tube. One milliliter of 500 mL/L polyethylene glycol (PEG) (Sigma) was added to the cells by drops for 1 min. Nine milliliters of serum-free RPMI medium 1640 was added to the mixture for 10 min. The cells were pelleted by centrifugation at 500 g for 5 min. The supernatant was removed and the cells were resuspended in 5 mL complete DC medium and plated in a T25 flask that was incubated at 37 °C in 50 mL/L carbon dioxide.
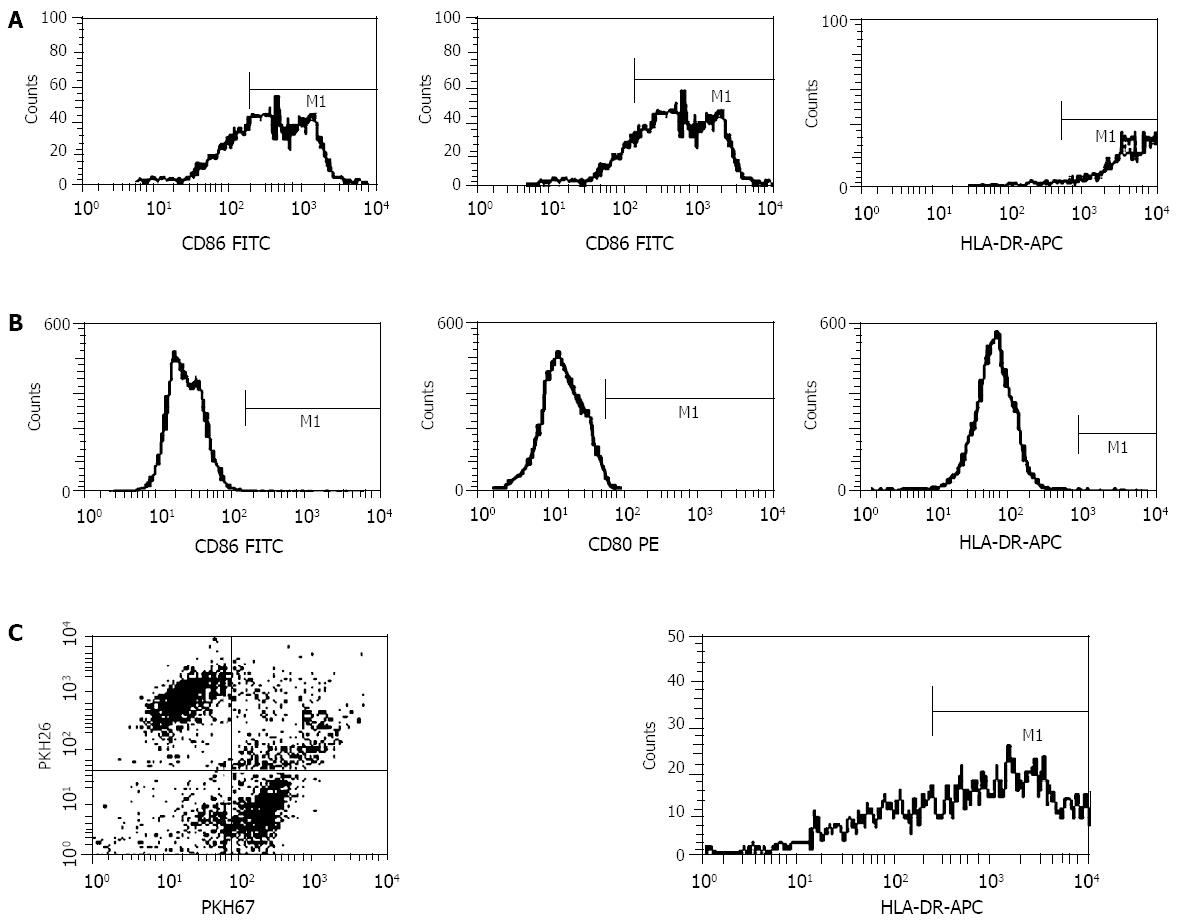
Fusion efficacy was evaluated by fluorescence microscopic analysis and flow cytometry analysis using Facsort (Becton Dickinson). DCs were washed with PBS and incubated with murine antibodies HLA-DR-APC, CD80-PE, CD86-FITC (PharMingen) for 15 min at 4 °C. Fusions were washed with PBS and incubated with murine antibody HLA-DR-APC for 15 min at 4 °C. Samples were then washed, fixed with 10 g/L paraformaldehyde, and subjected to flow cytometry analysis.
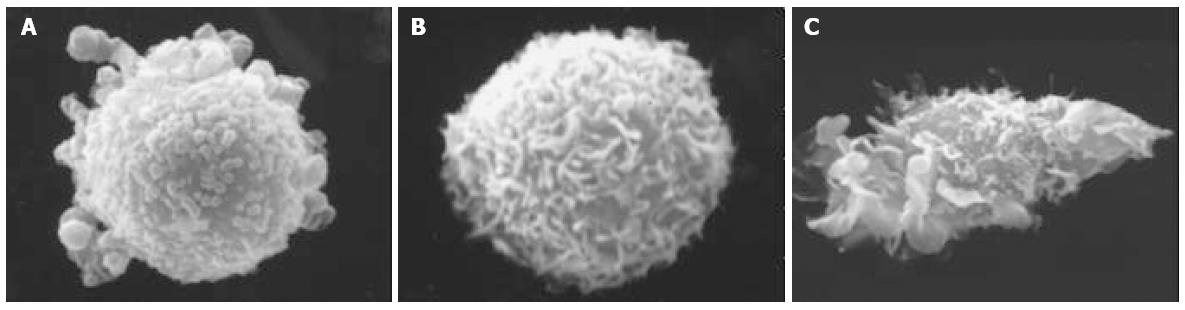
Cells were fixed with 12 g/L glutaraldehyde in 0.1 mol/L PBS (pH7.4). Fixed cells were coated with 1 g/L poly-L-lysine, dehydrated in ascending concentrations of ethanol, treated with isoamyl acetate, and critical-point dried with liquid CO2. Specimens were coated with vaccum-evaporated, iron-spattered gold and observed with a S-2250N scanning electron microscope (HITACHI).
Dendritomas obtained by using 500 mL/L PEG were mixed with CD8+ T lymphocytes at a ratio of 1:10. CD8+ cells to be used were pelleted by centrifugation at 500 g for 5 min and resuspended in 1 mL medium containing RPMI-1640, 10% human serum, 5 ng/mL IL-12, and 20 U/mL IL-2. The mixture was incubated at 37 °C in 50 mL/L carbon dioxide for 8 d. As controls, homologous DCs were mixed with CD8+ T cells at the same ratio.
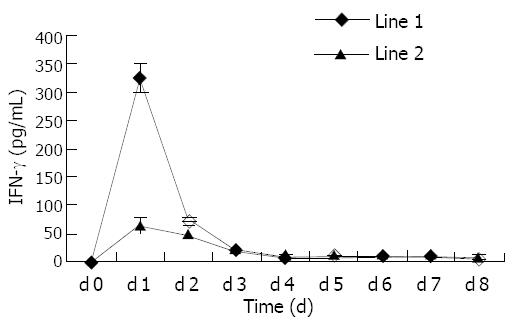
Each day CTL-generating cultures were refed. The supernatants of cultures were harvested and frozen at -20 °C. Interferon-γ (IFN-γ) assay was performed on the supernatants by a commercially available ELISA kit (R&D). Each assay was performed according to the manufacturer’s instructions. The lower detection limit was 4 pg/mL. All samples and standards were run in triplicate.
To determine whether dendritomas stimulated a tumor cell-specific CTL response, cytotoxicity assay was performed using the cultured tumor cells as target cells. CTL effector cells and tumor cells were mixed in a 24-well tissue culture plate with a round-bottom at the concentration of 100:1 effectors-to-target cells. The mixtures were incubated at 37 °C in 50 mL carbon dioxide for 2 d. Tumor apoptosis was measured using an Annexin V-FITC/PI kit (Clontech) by FACS analysis after the mixtures were cultured for 24 h and 48 h respectively. The tumor cells were detached and incubated with Annexin V-FITC for 30 min at 4 °C after the unattached CTLs were removed by magnetic microbeads. The samples were washed 3 times with PBS and measured by FACS analysis. To determine whether the lysis was tumor cell –specific, mammary cancer cells SK-BR-3 (ATCC#HTB-30) and ovarian cancer cells SK-OV-3 (ATCC#HTB-77) were used as target cells in similar cytotoxicity assay.
On scanning electron microscopy, SW480 cells had short processes on a plain cell surface and DCs had long denritic processes. Dendritomas were formed by fusions of the dendritic cells with CC cells (Figure 1). Fusion efficacy was about 16%. It was assessed by the analysis of fusions of SW480 cells stained with red fluorescent dye and DCs stained with green fluorescent dye using a fluorescent microscope (Figure 2) and flow cytometry repectively (Figure 3C).
To determine whether human DCs could be used in the generation of heterokaryons with tumor cells, DCs from PBMC of CC patients were prepared. Flow cytometry demonstrated that DCs highly expressed CD80, CD86 and HLA-DR, but SW480 cells did not. Dendritomas, which showed dual red and green fluorescence, highly expressed the major molecules HLA-DR of DCs (Figure 3).
To determine whether dendritomas effectively presented tumor antigens to effective cells, CD8+ T lymphocytes were purified from autologous peripheral blood and cocultured with dendritomas at a ratio of 10:1. Trypan blue exclusion test showed that, 6 d after the stimulation, CTLs were activated to proliferate and the mumber of T cells increased (from 1.2×107 to 1.8×107). After activation, CTLs secreted high levels of IFN-γ, and the secretion of this cytokine induced by dendritomas was higher than that of controls (Figure 4).
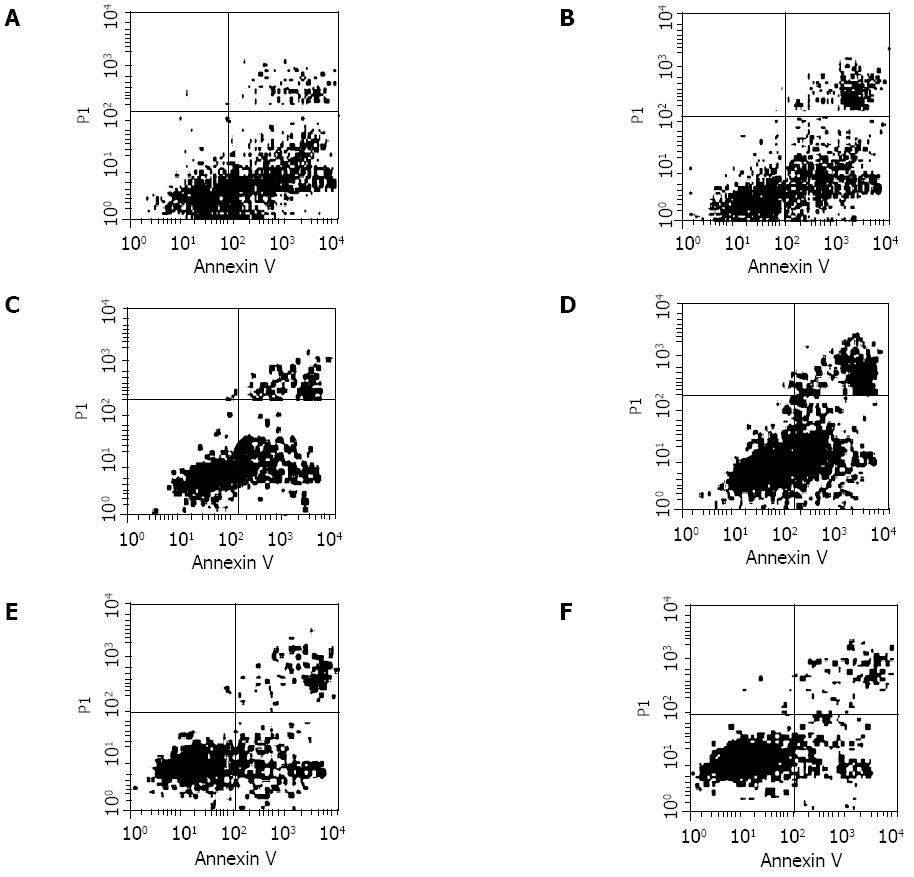
To investigate whether the CTLs induced by dendritomas, had a tumor-specific response, CTLs and tumor cells were mixed in a 24-well tissue culture plate with a round-bottom at the concentration of 100:1 effectors-to-target cells. Tumor apoptosis was measured using an AnnexinV-FITC/PI kit by FACS analysis after the mixtures were cultured for 24 h and 48 h respectively. The result indicated that the lysis was autologous tumor specific (Figure 5). The early apoptotic ratio of colon cancer SW480 cells was higher than that of controls, which was affected by cytotoxic T lymphocytes (CTL) that activated by denritomas after co-cultured for 24 h. Although there were no differences in the apoptotic ratios of tumor cells after co-cultured for 48 h, the necrotic fragments of colon cancer were higher than those of controls.
Dendritic cells are professional antigen-presenting cells that play a vital role in stimulating immune responses. Not only can they activate naïve CD4+ T helper cells, but they also stimulate unprimed CD8+ cytotoxic T lymphocytes[1,17,18]. Many studies have shown that DCs, when effectively loaded with or expressing tumor antigens, can activate antitumor immune responses through cellular and humoral actions[19]. However, for those tumors whose tumor antigens have not been identified, especially for primary tumors from patients, fusion between DCs and tumor cells presented a promising alternative strategy. Because the process of isolating cells from primary tissues in gastrointestinal tumor was time-consuming and prone to contam- ination, these considerations have limited the clinical application of this approach. In this study, an attempt was made to fuse DCs with human colon cancer SW480 cells to induce a colon cancer-specific antitumor immune response, because there were some common antigens between primary tumor cells and homogenous cell line.
It is important to determine the fusion efficacy of DCs and tumor cells by treatment with PEG. Two-color FACS analysis showed that approximately 100 mL/L to 160 mL of PEG-treated non-adherent cells were positive for both PKH67-GL (DCs were fluorescently stained) and PKH26-GL (SW480 cells were fluorescently stained) in our 5 separate CC patients, which highly expressed the MHC of DCs for antigen presentation (Figure 3C). These results were consistent with those of the landmark kidney cancer trial[20]. It is conceivable, therefore, that the fusions are able to present tumor antigen (s) to naive T cells by means of DC capability. Although exceptionally high fusion efficiencies sometimes have been reported using PEG, such reports might reflect an overlying optimistic interpretation of fluorescence-activated cell sorting data or nonrepresentative experiments[21]. Because fusions could generate not only tumor-DC hybrids but also tumor-tumor hybrids, DCs and tumor cells were fused at a ratio of 3:1 to decrease the tumor-tumor hybrids. Two types of aliphatic fluorescent dyes, PKH-67GL and PKH-26GL, have been widely used to label viable cells for in vitro and in vivo cell tracking[22,23]. In addition, researches have shown that there were no significant effects of the two dyes on cell viability, growth, or function[24,25].
To determine whether dendritomas effectively presented tumor antigens to immune cells and activated T lymphocytes, CD8+ T cells were isolated, and in vitro stimulation was performed. Then IFN-γ, a well-known marker of T-cell activation, was measured. Results showed that the secretion of CTLs activated by dendritomas was higher than that of controls after stimulation. Fusion hybrid vaccines might be more effective than other DC-based strategies because of superior antigen presentation[26]. Although DCs have a consistant capacity of processing exogenous antigens to achieve a major histocomp- atibility complex class II-restricted antigen presentation, the major histocompatibility complex I-restricted antigen presentation is often difficult to demonstrate when DCs are pulsed with complex protein antigens rather than with synthetic 8-or 9-mer peptides. Tumor-DC fusion potentially confers not only DC functionality but also a continuing source of endogenous tumor antigens for major histocompatibility complex class I presentation[21].
In the early stages of apoptosis, which occurs at the cell surface, one of these plasma membrane alterations is the translocation of phosphatidylserine (PS) from the inner side of the plasma membrane to the outer layer, by which PS becomes exposed at the external surface of the cell. Annexin V is a Ca2+ dependent phospholipid-bind-ing protein with high affinity for PS. Hence this protein can be used as a sensitive probe for PS exposure upon the cell membrane. So Annexin V assay offers the possibility of detecting early phases of apoptosis before the loss of cell membrane integrity and permits measurements of the kinetic of apoptotic death in relation to the cell cycle. More extensive FCM allows discrimination between different cell subpopulations that may or may not be involved in the apoptotic process. In comparison with the traditional tests, Annexin V assay was sensitive and easy to perform[27]. To determine whether CTLs could lyse tumor cells, CTLs were harvested and apoptosis assay was performed using tumor cells as target cells. To confirm that the CTL activity was tumor cells specific, mammary cancer cells SK-BR-3 and ovarian cancer cells SK-OV-3 were used as target controls, in addition to colon cancer cells. The results indicated that the lysis was tumor cell specific (Figure 5). Similar results were obtained in the 5 cases.
Several preclinical studies have shown that vaccines consisting of such hybrids can provide effective active immunization against animal tumors and specific in vitro sensitization of human T cells to relevant tumor antigens[10,11]. Furthermore, in contrast to other vaccine strategies, the tumor-DC fusion strategy has already been met with resounding clinical success when applied to the treatment of patients with advanced renal cancer[20]. Immunization with fusions of DCs and human colon cancer cells may be a promising method for the prevention and treatment of micrometastasis and recurrence after operation of CC.
We thank Dr. Xiang-Bai Chen (Univ. Pittsburgh, USA) for critically reading the manuscript. This work was supported by Technology Foundation of the Ministry of Education (China).
Edited by Zhao M and Wang XL Proofread by Xu FM
| 1. | Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3311] [Cited by in RCA: 3400] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 2. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10725] [Article Influence: 397.2] [Reference Citation Analysis (0)] |
| 3. | Gong J, Chen L, Chen D, Kashiwaba M, Manome Y, Tanaka T, Kufe D. Induction of antigen-specific antitumor immunity with adenovirus-transduced dendritic cells. Gene Ther. 1997;4:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, Rosenberg SA, Hwu P. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 675] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 626] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 7. | Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247-1256. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Ribas A, Butterfield LH, McBride WH, Jilani SM, Bui LA, Vollmer CM, Lau R, Dissette VB, Hu B, Chen AY. Genetic immunization for the melanoma antigen MART-1/Melan-A using recombinant adenovirus-transduced murine dendritic cells. Cancer Res. 1997;57:2865-2869. [PubMed] |
| 9. | Song ES, Lee V, Surh CD, Lynn A, Brumm D, Jolly DJ, Warner JF, Chada S. Antigen presentation in retroviral vector-mediated gene transfer in vivo. Proc Natl Acad Sci USA. 1997;94:1943-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 427] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci USA. 2000;97:2715-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516-5524. [PubMed] |
| 13. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |
| 14. | Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Zhang JK, Li J, Zhang J, Chen HB, Chen SB. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma in vivo. World J Gastroenterol. 2003;9:479-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Mehta-Damani A, Markowicz S, Engleman EG. Generation of antigen-specific CD8+ CTLs from naive precursors. J Immunol. 1994;153:996-1003. [PubMed] |
| 18. | Porgador A, Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 276] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Shurin MR. Dendritic cells presenting tumor antigen. Cancer Immunol Immunother. 1996;43:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Müller CA, Becker V. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 417] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | Shu S, Cohen P. Tumor-dendritic cell fusion technology and immunotherapy strategies. J Immunother. 2001;24:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Horan PK, Slezak SE. Stable cell membrane labelling. Nature. 1989;340:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 258] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Horan PK, Melnicoff MJ, Jensen BD, Slezak SE. Fluorescent cell labeling for in vivo and in vitro cell tracking. Methods Cell Biol. 1990;33:469-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877-11882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 425] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Oh DJ, Lee GM, Francis K, Palsson BO. Phototoxicity of the fluorescent membrane dyes PKH2 and PKH26 on the human hematopoietic KG1a progenitor cell line. Cytometry. 1999;36:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Hart I, Colaco C. Immunotherapy. Fusion induces tumour rejection. Nature. 1997;388:626-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluo-rescein labelled Annexin V. J Immunol Methods. 1995;184:39-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3792] [Cited by in RCA: 4023] [Article Influence: 134.1] [Reference Citation Analysis (0)] |