INTRODUCTION
Angiogenesis is the development of new blood vessels from an existing vascular network. It is mostly related to pathological processes (wound healing, cardiac ischemia, diabetic retinopathy, and tumour growth and metastasis). Usually a developing tumour does not reach a threshold size of around 1-2 mm3 as lack of oxygen and nutrients and accumulation of waste products. Blood vessels in tumour are usually disorganized and lack of structural integrity and prone to collapse, which result in areas of inadequate perfusion and transient hypoxia.
However, in order for a macroscopic tumour to grow, adequate oxygen delivery must be effected via tumour angiogenesis that results from an increased synthesis of angiogenic factors and a decreased synthesis of anti-angiogenic factors. The metabolic adaptation of tumour cells to reduced oxygen availability by increasing glucose transport and glycolysis and shif the balance between pro- and anti-apoptotic factors to promote survival are also important consequences in response to hypoxia. In this regard, HIF-1, induced by many factors is mainly implicated in tumour angiogenesis. This review mainly sets out to summarize the regulators of HIF-1α and signaling pathways involved based on its structure and function.
HYPOXIA AND HIF-1
Hypoxia is one of the major drivers to tumour progression as hypoxic areas form in human tumours when the growth of tumour cells in a given area outstrips local neovascularization, thereby creating areas of inadequate perfusion. Although several transcriptional factors have been reported to be involved in the response to hypoxic stress such as AP-1, NF-κB and HIF-1, HIF-1 is the most potent inducer of the expression of genes such as those encoding for glycolytic enzymes, VEGF and erythropoietin[1-3].
HIF-α subunit exists as at least three isoforms, HIF-1α, HIF- 2α and HIF-3α. HIF-1α and HIF-2α can form heterodimers with HIF-β. Although HIF-β subunits are constitutive nuclear proteins, both HIF-1α and HIF-2α subunits are strongly induced by hypoxia in a similar manner. HIF-1α is up-regulated in hypoxic tumour cells and activates the transcription of target genes by binding to cis-acting enhancers, hypoxic responsive element (HRE) close to the promoters of these genes with a result of tumour cellular adaptation to hypoxia and tumour angiogenesis, and promotion of further growth of the primary tumour. Studies have shown HIF-1α to be over-expressed by both tumour cells and such stromal cells as macrophages in many forms of human malignancy[4,5].
STRUCTURE OF HIF
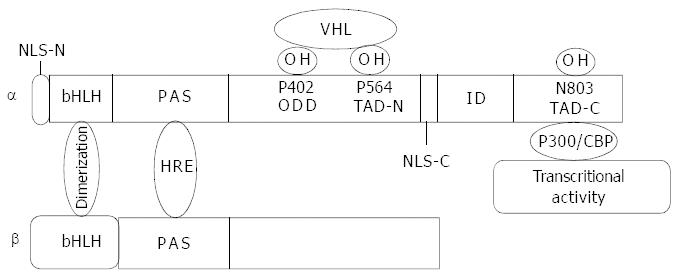
Both of the two subunits of HIF belong to a family of bHLH-PAS. Interactions between HLH-PAS domains from the two subunits mediate their dimerization, and individual basic regions of the two subunits then make contact with their corresponding DNA sequences, namely HRE. Specific interactions between three amino acids (Ser22, Ala25, Arg30) of the HIF-1 subunit and DNA bases were identified[6]. However, a major contribution towards the structure of HIF is attributed to a structure-function analysis of HIF-1α, which has revealed: (1) The N-terminal half of the molecule (amino acids 1-390) containing the bHLH-PAS domain was required for dimerization and DNA binding[7]. (2) The domains in C-terminal were required for hypoxia-induced nuclear localization, protein stabilization and transactivation[8]. Further study suggested that amino acids 401-603 of HIF-1α constituted an oxygen-dependent degradation domain (ODD), as presence or absence of this region had significant effects on the protein stability under non-hypoxia conditions[9]. (2) There are two nuclear location signals (NLS) located within N-terminal (amino acids 17-33) and C-terminal (amino acids 718-721) respectively. However, only the one within C-terminal was responsible for inducible nuclear accumulation of HIF-1α[10]. (4) HIF-1α has been found to contain two transactivation domains (TAD) at the C-terminal (amino acids 531-575 and 786-826)[8]. The transcriptional activation domains were separated by an amino acid sequence (amino acids 575-786) that inhibits transactivation (Figure 1)[8].
Figure 1 Molecular structure of HIF-1α and HIF-1β.
bHLH domain mediates dimerization of the two subunits. PAS domain is responsible for DNA binding. Proline residues of 402 and 564 at ODD domain are hydroxylized by proline hydroxylase and recog-nized by VHL and then targeted to the ubiquitin proteasome pathway. Asn803 at the C-terminal transactivation domain (TAD-C) is hydroxylized by FIH-1 (factor inhibiting HIF-1) with a result of inhibition of HIF-1α interaction with co-activator p300 and conse-quently inhibits transcriptional activity. The nuclear location signal at C-terminal functions in HIF-1α translocation into nuclei.
REGULATION OF HIF-1
The first regulator of HIF-1 is oxygen. HIF-1α appears to be the HIF-1 subunit regulated by hypoxia. The oxygen sensors in the HIF-1α pathway are two kinds of oxygen dependent hydroxylases. One is prolyl hydroxylase which could hydroxylize the proline residues 402 and 564 at the oxygen dependent domain (ODD) of HIF-1 in the presence of oxygen and iron with a result of HIF-α degradation[11]. The other is hydroxylation of Asn803 at the C-terminal transactivation domain (TAD-C) by FIH-1, which could inhibit the interaction of HIF-1α with co-activator p300 with a subsequent inhibition of HIF-1α transactivity[12]. Chan et al employed a novel hydroxylation-specific antibody to detect hydroxylized HIF-1α and their study further confirmed the hydroxylation of proline 564 at ODD of HIF-1α under normoxia[13].
Oncogene comes as the second regulator. Many oncogenes have effects on HIF-1α. Among them, some function in regulation of HIF-1α protein stability or degradation, others play roles in several activated signaling pathways. Tumour suppressor genes as p53 and von Hippel-Lindau (VHL) influence the levels and functions of HIF-1. The wild type (wt) form of p53 protein was involved in inhibiting HIF-1 activity by targeting the HIF-1α subunit for Mdm2-mediated ubiquitination and proteasomal degradation[14], and in inducing inhibitors of angiogenesis such as thrombospondin-1[15], while loss of wt p53 (by gene deletion or mutation) could enhance HIF-1α accumulation in hypoxia, and augment HIF-1 dependent expression of VEGF in tumour cells[16]. The pVHL gene product could also regulate the stability of HIF-α for oxygen-dependent proteolysis[17]. In the presence of oxygen, pVHL could bind to and target prolyl hydroxylized HIF-α subunits to the ubiquitin proteasome pathway[18]. This process involved interaction between conserved sub-sequences within the oxygen-dependent degradation domains of HIF-α subunits and the β-domain of pVHL, with pVHL acting as the recognition element of a multicomponent E3 ubiquitin ligase[19]. Furthermore, in VHL-deficient cells, Pro-564 in HIF-1α had a detectable amount of hydroxylation following transition to hypoxia, indicating that the post-translational modification is not reversible[13]. In addition, inactivation of other tumour suppressors such as PTEN which is an antagonist of PI-3K signaling could function by removing phosphate at the 3rd position of phosphatidylinositol biphosphate and triphosphate. Loss of the tumour suppressor function of PETN would augment HIF-1 mediated gene expression[20] and restoration of PTEN could inhibit the expression of HIF-1α[21]. Amplification of Akt1 and Akt2 is quite common in human tumours which express a high level of HIF-1α. While activation of a variety of oncogenes and growth signaling pathways can induce the HIF system in non-hypoxic cells or amplify the response to hypoxia. Indeed, introduction of v-Src or RasV12 oncogenes resulted in stabilization of normoxic HIF-1α[17] and loss of hydroxylated-Pro-564 demonstrated that oncogenes induced stabilization of HIF-1α signals by inhibiting prolyl hydroxylation[13].
The third regulator is a battery of growth factors and cytokines from stromal and parenchymal cells such as EGF[22], transforming growth factor-α[23], insulin-like growth factors 1 and 2[24], heregulin[25], and interleukin-1β[26] via autocrine and paracrine pathways. These regulators not only induce the expression of HIF-1α protein, HIF-1 DNA binding activity and transactivity, but also make HIF-1 target gene expression under normoxia or hypoxia. In addition, some of the growth factors are HIF-1 target genes such as IGF-2, IGF-BP1, 2 and 3, and TGF-α as well as VEGF, which make HIF-1 contribute to autocrine-signaling pathways.
The fourth one is a group of reactive oxygen species (ROS) resulting from carcinogens such as Vanadate[27] and Cr (VI)[28] or stimulation of cytokines such as angiotensin[29] and TNFα[30]. However, it seems controversial when it comes to the production of ROS under hypoxia and their individual role in regulation of HIF-1α. Some studies have provided experimental evidence in supporting the hypothesis that ROS generated from mitochondria increased under hypoxia and is required for HIF-1 activity and transcription of its downstream target genes[31,32]. An alternative model proposed that hypoxia resulted in decreased production of ROS due to nicotinamide adenine dinucleotide phosphate (NADPH) oxidases[33]. It is well known that ROS plays an important role in carcinogenesis induced by a variety of carcinogens. This was examplified by Cr(VI) complex[28], which could induce HIF-1α activation and stabilization via ROS including O2-, H2O2, and OH[28], suggesting that HIF-1α may play its role in carcinogenesis.
HIF-2α, a homologue of HIF-1α initially described as endothelium and fetus specific and named endothelial PAS protein-1/HIF-related factor/HIF-like factor, has also been cloned and shown to form a transcriptionally active complex with ARNT by transient transfection assay[34]. Structurally and functionally HIF-2α is highly similar to HIF-1α, yet exhibits more restricted tissue-specific expression.
It has been shown that HIF-3α also exhibits conservation with HIF-1α and HIF-2α in HLH and PAS domains, but does not possess a hypoxia-inducible domain[35] and might function primarily as an inhibitor of HIF-1α[36].
SIGNALING PATHWAYS INVOLVED IN REGULATION OF HIF-1
HIF-1 is a phosphorylated protein and its phosphorylation is involved in HIF-1α subunit expression and/or stabilization as well as in the regulation of HIF-1 transcriptional activity. Three signaling pathways involved in the regulation of HIF-1α have been reported to date.
The PI-3k pathway has been mainly and frequently implicated in regulation of HIF-1α protein expression and stability[26], although it was also involved in regulation of HIF-1α transcription in a couple of studies[37]. PI-3K is activated by ligation of a variety of growth factors to their cognate receptor tyrosine kinases with a subsequent phosphorylation and activation of its downstream signaling pathways such as a serine-threonine protein kinase Akt (protein kinase B) and FRAP (FBKP-12 rapamycin associated protein, also known as mammalian target of rapamycin) pathway.
Akt is also activated by hypoxia, yet its mechanism is still not clear. Activated Akt initiates two different pathways in regulation of HIF-1α. The function of these two pathways appears to show consistent impact on HIF-1α activation. In this regard, phospho-Akt either inhibits the function of GSK, a downstream target of Akt, via phosphorylation with a result of increased HIF-1α stability under hypoxia in a time dependent manner[22] or protein synthesis[38,39], or activates FRAP pathway resulting in an increased HIF-1α synthesis[40].
A recent study performed by Sodhi showed that there was a potential consensus site in the oxygen-dependent degradation domain of HIF-1α for GSK3β and that this site could play a role in the regulation of HIF-1α protein stability, highlighting the effect of PI-3K/Akt/GSK pathway on HIF-1α[41]. It was further confirmed by co-transfection of an HIF-1 reporter plasmid either with the p85 or with the Akt dominant negative vector that the disruption of the PI-3K/Akt pathway impaired the activation of HIF-1 as well as VEGF gene transcription in hypoxic NIH3T3R cells[20]. Besides, in a renal cancer cell line mutant for VHL, HIF-1α was constitutively stabilized, and the PI-3K inhibitor still down-regulated HIF-1α expression, suggesting a novel pathway for HIF-1α protein regulation independent of VHL[17]. HIF-1α expression induced by EGF and insulin or loss of function of PTEN through PI-3K pathway[20,22,42] provided an additional evidence.
In contrast, an alternative study showed that although serum stimulation could induced a slight accumulation of HIF-1α protein in a PI-3K/Akt pathway dependent fashion, hypoxia induced much higher levels of HIF-1α protein and HIF-1 DNA binding activity independent of PI-3K and mTOR activity and high levels of Akt signaling could modestly increase HIF-1α protein, but this increase did not affect HIF-1 target gene expression. Besides the effects of constitutively active Akt on HIF-1 were cell type specific, drawing a conclusion that PI-3K/Akt pathway was not exclusive for hypoxic induction of HIF-1 subunits or activity, and constitutively active Akt was not itself sufficient to induce HIF activity[43], suggesting that the involvement of PI-3K/Akt pathway in regulation of HIF-1α is a much more complicated process which not only exhibits a cell type- and stimulus type- dependency, but also may have a cross talk with other unknown signaling pathways.
The Raf-1/MEK1/ERK pathway appears mainly to regulate HIF-1 transactivation and DNA binding activity and the C-terminal domain of HIF-1α encompassing the transactivation domains of the protein, was demonstrated to be directly phosphorylated by ERK1 by an in vitro kinase assay[44]. Although HIF-1α phosphorylation was not required for HIF-TAD/p300 interaction, MAPK was required for the transactivation activity of HIF-1α. Furthermore, inhibition of MAPK could disrupt the HIF-p300 interaction and suppress the transactivation activity of p300[45] and overexpression of MEK1, an upstream ERK activator, could stimulate the transactivation of both p300 and HIF-1α[45]. Reporter gene assays and EMSA experiments in vitro have demonstrated that the MEK1 inhibitor PD98059 or ERK dominant negative mutants together with an HIF-1 reporter gene were able to block the transcriptional activity of HIF-1 without affecting its DNA binding activity[28,44,46].
Mammalian cells respond to a variety of noxious stimuli such as chemicals, radiation, osmotic shock, and hypoxia by induction of stress activated protein kinases (SAPKs/JNK). Among them, both of p38 and JNK1 play critical roles in responding to cellular stress and promoting cell growth and survival. JNK1 and p38 are also serine/threonine protein kinases that phosphorylate nuclear transcriptional factors which regulate target genes in response to cellular stress. It has been shown that JNK1 and p38 were activated by hypoxia and involved in induction of HIF-1α expression[47]. Additional evidence indicated that HIF-1α phosphorylation by p38 could be involved in inhibition of the inhibitory domain located within the C-terminal region of HIF-1α[48]. Another study showed that p38 signaling mediated HIF-1α and VEGF induction by Cr (VI) in DU145 human prostate carcinoma cells[28].
In addition, a calmodulin dominant negative mutant and W7, a calmodulin antagonist, as well as BAPTA, an intracellular calcium chelator, inhibited hypoxia-induced HIF-1 activation. A MEKK1 (a kinase upstream of JNK) dominant negative mutant had no effect. Moreover, BAPTA, calmidazolium, a calmodulin antagonist and PD98059 inhibited VEGF secretion in hypoxic HepG2 cells. These results indicate that elevated calcium in hypoxia could participate in HIF-1 activation, suggesting that calcium and calmodulin act on the upstream of ERK in the hypoxia signal transduction pathway[28].
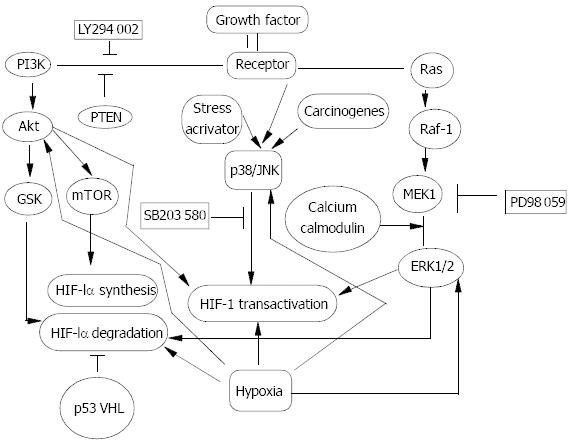
One study in T47D cell indicated PI-3K/Akt was not activated under hypoxic condition[17]. While our study in the same cell line showed that ERK1/2 was not activated by hypoxia either. However, bFGF activated PI-3K/Akt, ERK1/2 and p38 pathways both under normoxia and hypoxia. Under hypoxia, bFGF synergized with hypoxia in regulation of HIF-1α protein expression and transactivation. While under normoxia, bFGF increased HIF-1α protein synthesis and transactivation depending on PI-3K/Akt and ERK1/2 pathways respectively. p38 pathway had no effect on HIF-1α and VEGF. Our result further confirmed the role of PI-3K/Akt and ERK1/2 in regulation of HIF-1α was induced by growth factors and that activation of PI-3K/Akt and ERK1/2 by hypoxia was not a universal phenomenon (Figure 2).
Figure 2 Signal transduction pathway in HIF-1α regulation.
Oncogenes, growth factors and hypoxia have been documented to regulate HIF-1α protein and increase its transactivity. PI-3K/Akt, Raf-1/MEK/ERK1/2 and p38/JNK pathways were activated in response to oncogenes, growth factors and stress activators such as hypoxia, and carcinogens to different ex-tent in a cell type- and stimulus type-specific manner. GSK and mTOR were two target events of Akt and could contribute to decreasing HIF-1α degradation and increasing HIF-1α pro-tein synthesis. Activated ERK1/2 could mainly up-regulate
HIF-1α transcriptional activity. Calcium and Calmodulin could act on HIF-1α indirectly as upstream events of ERK1/2. While the effect of p38/JNK pathway on HIF-1α is controver-sial depending on stimulus and cell type. Some oncoproteins themselves are inter-events of those signal pathways. Amplifi-cation of p110, a subunit of PI-3K, Akt and loss of PTEN could contribute to activation of PI-3K pathway, while mutation of RAS and amplified Raf-1 and ERK1/2 could activate the extra-cellular-signal regulated kinase (ERK1/2). In addition, muta-tion of p53 and loss of VHL could decrease HIF-1α degradation, although the signal pathways involved are poorly understood.
HIF-1α, ANGIOGENESIS AND TUMOUR PROGNOSIS
HIF-1 has been taken as a key factor in regulation of VEGF and VEGFR and other angiogenic factors. Immunohistochemical analysis of human tumour biopsies revealed that dramatic overexpression of HIF-1α was seen in common cancers[49] and associated with tumour VEGF expression and vascularization[50,51]. Constitutively expressed VEGF increased the number of new capillaries around hepatic sinuses[52]. HIF-1 activity has also been manipulated in human cancer cell lines. Expression of VEGF, xenograft growth and angiogenesis were markedly increased in HCT116 colon cancer cells transfected with an expression vector encoding HIF-1α[53]. In addition, emerging evidence has suggested that loss or gain of HIF-1 activity is negatively and positively correlated respectively with tumour growth and angiogenesis[50,54-56].
VEGF overexpression in several tumours has been correlated with high vascularity, lymph node metastasis, and liver metastasis, and a poorer prognosis than VEGF-negative tumours[57,58] and a direct relationship between angiogenesis and metastasis in a large and diverse array of other tumours, including melanomas, gliomas, cancers of the lung, bladder and prostate, and many others[59,60] was established. Many reports have shown that intratumoural microvessel density (IMD) is a significant and independent prognostic indicator in human breast cancer.
In a study of ovarian cancer, HIF-1α expression was correlated with apoptosis in most tumours. Besides, according to the identification of a cis-acting hypoxia-response element containing an HIF-1 binding site, a variety of proteins targeted by HIF-1 were involved in tumour cell proliferation, survival, adhesion and mobility, implicating the role of HIF-1 in these processes. A correlation of a reduced inducibility of HIF-1α and HIF-2α and an increased survival of the breast cancer cell lines under hypoxia was reported[61].
In addition, HIF-1α overexpression in combination with deficiency or mutation of tumour suppressor genes such as VHL, p53 and PTEN, and amplification of oncogenes (Akt, Ras, ERK1/2) was frequently seen in human cancer and these genetic alterations have been associated with tumour growth, invasion and metastasis. Clinically, a high level of HIF-1α expression had an association with a decreased overall survival and a disease-free survival in lymph-node-negative breast cancer[62] and an increased mortality in early stage cervical carcinoma[63] and lymph-node-negative breast cancer[50]. Furthermore, HIF-1α overexpression was associated with treatment failure and/or mortality in nasopharyngeal squamous cell cancer[64], suggesting a potential role of HIF-1α in tumour prognosis. Finally, the findings that HER-2/neu immunoactivity and gene amplification, VEGF expression, and Ki-67 expression were correlated strongly with HIF-1α positivity in lymph node negative breast carcinoma[62] provided a further evidence. All data above support a hypothesis that the presence of hypoxia in a tumour reflects a poor prognosis[65].
SUMMARY
HIF-1 activation is regulated through different mechanisms from HIF-1α subunit stabilization[18,46], phosphorylation[44], modification of redox conditions to interaction with coactivators[45,66] involving several signal transduction pathways including PI-3K, MAPK (ERK1/2) and p38 pathways. However, it appears that these mechanisms and signaling pathways can be cell-type specific and stimulus-specific. Hypoxia, oncogenes and a variety of growth factors and cytokines increase HIF-1α stability and/or synthesis and transactivation to initiate tumour angiogenesis, metabolic adaptation to hypoxic situation and promote cell survival or anti-apoptosis[67,68] resulting from a consequence of more than sixty putative direct HIF-1 target gene expressions. Taken together, HIF-1 is activated in tumour and plays a critical role in initiation of tumour angiogenesis, tumour growth, progression and metastasis.
The crucial role of HIF-1 in tumour angiogenesis has sparked scientists and clinical researchers to try their best to understand the whole diagram of HIF-1 so as to find out novel approaches to inhibit HIF-1 overexpression. Indeed, the combination of anti-angiogenic agent and inhibitor of HIF-1 might be particularly efficacious, as the angiogenesis inhibitor would cut off the tumour’s blood supply and HIF-1 inhibitor would reduce the ability of tumour adaptation to hypoxia and suppress the proliferation and promote apoptosis. Screens for small-molecule inhibitors of HIF-1 are underway and several agents that inhibit HIF-1, angiogenesis and xenograft growth have been identified. Full understanding of the regulators, and signal transduction pathways of HIF-1 and its interaction with other proteins is beneficial to the seeking of novel strategies to cure tumour and other angiogenic diseases.
Edited by Zhu LH and Wang XL Proofread by Xu FM