Published online Mar 15, 2004. doi: 10.3748/wjg.v10.i6.889
Revised: December 23, 2003
Accepted: January 8, 2004
Published online: March 15, 2004
AIM: To investigate the effect of cholesterol (Ch) on the growth and functional protein expression of rabbit bile duct fibroblasts.
METHODS: The cultured bile duct fibroblasts were divided randomly into two groups: the control group and the experiment group (fibroblasts were incubated respectively with 0.6 g/L Ch for 12, 24, 36 and 48 h). The growth and DNA synthesis of bile duct fibroblasts were measured by the means of 3H-TdR incorporation. The total protein content of fibroblast was measured by BSA protein assay reagent kit, then the expression of α-actin was analyzed semi-quantitatively by Western blot.
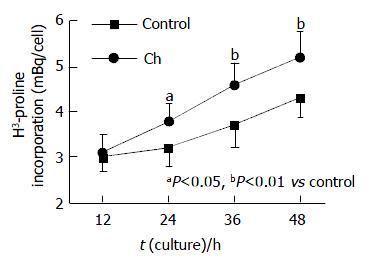
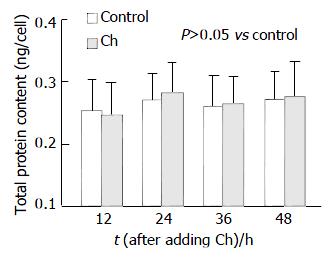
RESULTS: After treatment with 0.6 g/L Ch for 12, 24, 36 and 48 h, the values of 3H-TdR incorporation of bile duct fibroblasts were respectively 3.1 ± 0.39, 3.8 ± 0.37, 4.6 ± 0.48 and 5.2 ± 0.56 mBq/cell, and the values of the corresponding control groups were 3.0 ± 0.33, 3.2 ± 0.39, 3.7 ± 0.49 and 4.3 ± 0.43 mBq/cell. After comparing the values of experiment groups and their corresponding control groups, it was found that the 3H-TdR incorporation of bile duct fibroblasts after treatment with 0.6 g/L Ch for 24, 36 and 48 h were significantly increased (P < 0.05, P < 0.01, P < 0.01), while the 3H-TdR incorporation of 12-h group was not different statistically from its control group. Ch had no obvious effect on the total protein content of fibroblasts. After incubated with 0.6 g/L Ch for 12, 24, 36 and 48 h, the total protein content of each experiment group was not altered markedly compared with its corresponding control group. The values of experiment groups were 0.246 ± 0.051, 0.280 ± 0.049, 0.263 ± 0.044 and 0.275 ± 0.056 ng/cell, and those of corresponding control groups were 0.253 ± 0.048, 0.270 ± 0.042, 0.258 ± 0.050 and 0.270 ± 0.045 ng/cell. Western blot analysis revealed that the α-actin expression in fibroblasts affected by Ch for 12 and 24 h was not markedly changed compared with their corresponding control groups (P>0.05), the values of total gray scale of 12- and 24-h groups were 1 748 ± 185 and 1 756 ± 173, respectively. But after stimulation with Ch for 36 h, the total gray scale of fibroblasts (1 923 ± 204) was significantly higher than that of control group (1 734 ± 197). When the time of Ch treatment was lengthened to 48 h, the α-actin expression was markedly elevated, the total gray scale was 2 189 ± 231 (P < 0.01 vs control group).
CONCLUSION: Moderately concentrated Ch can promote the proliferation of bile duct fibroblasts at early stage. With the prolongation of Ch treatment, the α-actin expression of fibroblasts was also increased, but the hypertrophy of fibroblasts was not observed.
- Citation: Chen BY, Wei JG, Wang YC, Yu J, Qian JX, Chen YM, Xu J. Effects of cholesterol on proliferation and functional protein expression in rabbit bile duct fibroblasts. World J Gastroenterol 2004; 10(6): 889-893
- URL: https://www.wjgnet.com/1007-9327/full/v10/i6/889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i6.889
The disorder of cholesterol metabolism is an important cause of biliary diseases. Previous studies suggest that cholesterol can change the motility of cholecyst[1,2] and decrease gallbladder contraction[3-7] in the patients with cholesterol calculus and in the animals with hypercholesterolemia. Weak contraction of gallbladder may be a reason of cholesterol calculus[8-12]. Researchers consider that cholesterol metabolism disorder has an effect on the structure and function of bile duct and sphincter of bile duct (SBD)[13-16] . We have found that cholesterol liposome (CL) affected not only the configuration and quantity of cytoskeleton in rabbit SBD smooth muscle cells, but also the proliferation of cells[17,18]. There are many fibroblasts in biliary system except that SBD is formed mainly with smooth muscle cells[19-22]. In our previous experiment, we observed that middle concentration Ch could accelerate the proliferation of bile duct fibroblasts and result in the changes of phenotype[23,24]. Fibroblasts displayed some characteristics of myofibroblasts or smooth muscle cells[23,24]. In order to lucubrate the reactivity of bile duct fibroblasts to Ch and the role of fibroblasts on the configuration variation of bile duct and SBD, the effects of Ch on bile duct fibroblasts at different time point were studied and the relation between the effects of Ch and time was analyzed in this study.
New Zealand rabbits aged 1 month were provided by the Animal Center of the Fourth Military Medical University. Trysin (Gibco, Paisley, Renfrewshire, UK), Dulbecco’s modified eagles medium (DMEM) (Gibco, Paisley, Renfrewshire, UK), fetal calf serum (Qinghu Institute of Foetus Bovine Utilization in Jinhua Zhejiang), water soluble cholesterol (Sigma, St. Louis, USA), antibody of vimentin, α-actin and desmin (DakoCytomation, Glostrup, Denmark), ABC immunohistochemical kit (Shaanxi Biotech. Co., Xi’an, China), IMT-2 inverted biological microscope (Olympus Corporation, Japan), YJ-875 ltra-clean operating boar (Suhang Experimental Animal Technology Development Co), LD4-2 centrifugal machine (Beijing Medical Centrifugal Machine Factory), and BCA protein assay reagent kit (Pierce Chemical Company, Rockford, USA) were commercially obtained.
Ch was diluted to 0.6 g/L with DMEM before experiment. DMEM was dispensed according to the description. Fetal calf serum was inactivated for 30 min at 56 °C, and was stored at -20 °C. Trypsin was made into 2.5 g/L solution with PBS (0.01 mol/L, pH 7.4) and stored at 4 °C. TBST was prepared by mixing 10 mL of Tri-Cl, 8.78 g of NaCl, 500 μL of Tueen-20, and adding distilled water up to 1L.
Bile ducts of New Zealand rabbits were dissociated by the means of aseptic technique and broken by shears. The tissue was digested to become single cell suspension by trypsin (1.25 g/L). Cells were washed and resuspended with DMEM (containing 100 mL/L fetal calf serum) and incubated at room temperature for 75-90 min. Then the cells were collected and transferred into culture bottles. The cells of 2nd-4th passages were used for experiments.
Three glass cover slips (18 mm×18 mm) were placed into 6 cm diameter culture dishes, and then cell suspension was added and incubated at room temperature for 48 h. The slips covered with cells were washed twice with PBS (pH 7.4). Some slips were fixed by cold acetone for 15 min at 4 °C and used for HE staining, another three slips were fixed by citromint (40 g/L) for immunohistochemical ABC staining to exam vimentin and desmin expression.
Bile duct fibroblasts were planted in 96-well plates. Sub-confluent cells were cultured without serum for 24 h, then treated respectively by 0.6 g/L Ch for 12, 24, 36 and 48 h and pulsed with 18.5 kBq of [3H] thymidine for 4 h. The control group cells were incubated with DMEM containing 20 mL/L fetal calf serum instead of Ch. The radioactivity of each group was counted by Beckman LS6500 counter.
The cells of each group were trypsinized and counted. Cells were centrifugated at 1 000 r/min for 5 min at 4 °C, washed twice with ice-cold PBS and lysed in ice –cold lysis buffer for 30-60 min. The lysates were centrifugated at 12 000 g for 5 min at 4 °C and supernatant was transferred into new Eppendorf tubes. The standard curve was drawn according to the description of BCA protein assay reagent kit, and then the total protein content per cell was measured and converted.
Loading buffer was added to each lysate, which was subsequently boiled for 10 min. Equal amounts (10 µg) of cell extracts were separated by 100 g/L SDS-PAGE and transferred to nitrocellulose membrane. The membrane was blocked for one h at room temperature in 50 g/L skim milk and probed with α-actin antibody for one h. The membrane was washed three times with PBS-T and incubated for one h with secondary antibody. After washing the membrane with PBS-T for several times, the protein reactive to the primary antibody were visualized by electrochemiluminescence (ECL) detection, and semi-quantitatively analyzed by Kodak digital science 1D software.
Results were presented as mean ± SD. Significance was determined by Student’s t test or one-way ANOVA. P < 0.05 was considered statistically significant.
Under phase-contrast microscope, the cultured rabbit bile duct fibroblasts showed shuttle-shaped or multiangular. Their cytoplasm was clear and nucleus was large and ellipse, and their nucleoli were obvious. The isolated bile duct fibroblasts were free of smooth muscle cell contamination because they presented positive staining with vimentin and negative staining with desmin by the means of immunocytochemical ABC staining.
Following incubation with 0.6 g/L Ch for 12, 24, 36 and 48 h, the values of 3H-TdR incorporation of bile duct fibroblasts were respectively 3.1 ± 0.39, 3.8 ± 0.37, 4.6 ± 0.48 and 5.2 ± 0.56 mBq/cell, and those of the corresponding control groups were 3.0 ± 0.33, 3.2 ± 0.39, 3.7 ± 0.49 and 4.3 ± 0.43 mBq/cell. After comparing the values of experiment groups and their corresponding control groups, we found that the 3H-TdR incorporation of bile duct fibroblasts after treatment with 0.6 g/L Ch for 24, 36 and 48 h were significantly increased (P < 0.05, P < 0.01, P < 0.01), while the 3H-TdR incorporation of the 12 h group was not statistically significant as compared with the control group (Figure 1)
Ch has little effect on the total protein content of fibroblasts. After being incubated with 0.6 g/L Ch for 12, 24, 36 and 48 h, the total protein content of each experiment group was not altered markedly compared with its corresponding control group. The values of experiment groups were 0.246 ± 0.051, 0.280 ± 0.049, 0.263 ± 0.044 and 0.275 ± 0.056 ng/cell, and those of corresponding control groups were 0.253 ± 0.048, 0.270 ± 0.042, 0.258 ± 0.050 and 0.270 ± 0.045 ng/cell (Figure 2).
Western blot analysis revealed that the α-actin expression of fibroblasts affected by Ch for 12 h and 24 h was not markedly changed compared with their corresponding control groups, the values of total gray scale of the 12 h and 24 h groups were 1 748 ± 185 and 1 756 ± 173 respectively, but after stimulation with Ch for 36 h, the total gray scale of fibroblasts (1 923 ± 204) was significantly higher than that of control group (1 734 ± 197). When the time of Ch treatment was lengthened to 48 h, the α-actin expression was markedly elevated, and the total gray scale was 2 189 ± 231 (P < 0.01 vs control group) (Figure 3, Table 1).
Fibroblasts are derived from mesenchymal cell of embryo period. During the process of wound healing, fibroblasts can proliferate greatly by mitoses, and also synthesize and excrete collagen fibers and matrix components[25-29]. With the stimulation of trauma and other agents, some mature fibroblasts could change into infantile fibroblasts and their function could be recovered[30,31]. Cardiac fibroblasts are able to secret biological active substances, which facilitate the growth of myocardial cells[32,33] , suggesting that cardiac fibroblasts must play an important role in the normal growth of heart and its pathologic remodeling[34,35]. Hypoxia could, mediated by pulmonary arterial endothelial cells (PAECs), induce phenotype alteration of human embryonic lung fibroblasts, transforming to smooth muscle cell-like cells, suggesting that the transformation of human embryonic lung fibroblasts might be one of the reasons for nonmuscular lung arteriole to become muscular arteriole[36-38]. Silent fibroblasts in the border of wound can differentiate to contraction phenotype and have the special expression of α-SM actin. The cells are considered as myofibroblasts which can cause contraction of granulation tissue[39,40]. It is thus clear that fibroblasts can take part in many kinds of physiological and pathologic responses and they have important effects on the occurrence and development of diseases.
Biliary system is the unique passage of bile ejection, especially its terminal sphincter, SBD. Biliary system can modulate bile ejection and maintain normal pressure of biliary system[41,42]. The coordination of its anatomic structure and function makes it not only prevent regurgitation of duodenal fluid but also modulate and stabilize the pressure of bile duct[42-44]. So the structural remodeling of biliary system, especially the structural change and dysfunction of SBD, might be one of basic reasons of biliary system diseases occurrence. There are a lot of fibroblasts in bile duct system. In previous study, we have found that the middle concentration Ch can promote the proliferation of bile duct fibroblasts and make them present some phenotypic characteristics of muscle cell[23,24]. But at present, it is still unclear what are the exact effects of cholesterol on bile duct fibroblasts and how fibroblasts are revolved in remodeling of biliary system, especially what roles fibroblasts play in the remodeling of SBD. In order to lucubrate the problem, we observed the effects of Ch on the proliferation and functional protein expression of bile duct fibroblasts at different times and analyzed the relation between the effects of Ch and time.
3H-TdR, prosoma of DNA synthesis, can incorporate into DNA synthesis. So the radioactivity intensity of cells can reflect DNA metabolism and proliferation of cells. It has been demonstrated that middle concentration Ch can accelerate the proliferation of bile duct fibroblasts[23]. But the relation between Ch effects and time is still unknown. In the present study, our focal point is to observe how the Ch effects on fibroblasts alter with the changes of incubation time. Our results show that the DNA synthesis and proliferation of bile duct fibroblasts are elevated after incubated with moderately concentrated Ch for 24 h, and the effect becomes more significant gradually with the elongation of Ch treatment.
In the research of cardiovascular disease, it has been found that the proliferation and hypertrophy of cardiac fibroblasts participate in the cardiac remodeling[34,35]. In the process of estrogen-induced uterine enlargement, there are not only the hyperplasia of uterine smooth muscle cell and epithelial cell but also augmentation of size of cells. Chronic enteritis is linked with hypertrophy of intestinal smooth muscle cells[45-47]. From the phenomena mentioned above, we conjecture whether Ch can not only accelerate the proliferation of bile duct fibroblasts but also cause hypertrophy of fibroblasts. According to our result, the total protein content of bile duct fibroblasts was not altered after fibroblasts were treated with Ch, indicating that Ch might not significantly facilitate the hypertrophy of bile duct fibroblasts although it could promote the proliferation of fibroblasts greatly.
In our previous experiment, we detected that middle concentration Ch could increase α-actin expression of bile duct fibroblasts, and we also observed that bile duct fibroblasts showed some characteristics of muscle cells, which suggested that Ch might lead to the phenotypic variation of fibroblasts[24]. In present study, we aim to observe the time effects of Ch on fibroblasts by the means of Western blot. It has been demonstrated that α-actin expression in bile duct fibroblasts begins to increase after incubated with Ch for 36 h, and the effect becomes more significant after 48 h. From the results above, we can easily find that the proliferation of bile duct fibroblasts is enhanced after Ch treatment for 24 h, however, it is not until Ch incubation for 36 h that the α-actin expression in bile duct fibroblasts begins to ascend. It indicates that the short-term effects of Ch are mainly to promote the proliferation of bile duct fibroblasts, and by the prolongation of Ch treatment time, Ch can also alter the functional protein expression of fibroblasts. Ch has no obvious effect on the total protein content of bile duct fibroblasts, nevertheless, it can enhance α-actin expression in fibroblasts. It suggests that Ch can only result in the changes of some special protein expression instead of causing the hypertrophy of bile duct fibroblasts. The protein, α-actin, is an important functional protein existing in myofibroblasts and smooth muscle cells[48,49]. So we can realize that Ch accelerates the proliferation of bile duct fibroblasts, and what is most important is that Ch can induce bile duct fibroblasts to possess some phenotypic characteristics of muscle cell.
By studying the anatomy of SBD, people found that the length of SBD is 5-15 mm without accordant result and the data fluctuates in a wide range. It has not been lucubrated whether it is only attributed to the congenital diversity of individual or it is the result of SBD remodeling induced by some postnatal factors. Wei et al[21] have found that gallbladder-derived abdominal pain after cholecystectomy, recurrent bile calculus and cholangiectasis have significant correlation with too lengthy of SBD (≥10 mm). It has also been manifested that most of the patients whose SBD length exceeds 10 mm are often accompanied by SBD motor dysfunction[50]. Combining our present outcome, we suspect that some postnatal factors may result in constitution alteration of SBD, including the changes of length. The proliferation of fibroblasts, especially their phenotype transformation, may play an important role during the constitution changes of bile duct and SBD.
In conclusion, cholesterol does activate bile duct fibroblasts at the early stage, facilitating the proliferation of fibroblasts, and it can also induce the phenotype transformation of fibroblasts following the elongation of Ch treatment time. The alteration of fibroblasts might participate in the configuration remodeling of biliary system, especially the reconstitution of SBD. Gradually the function of bile duct system becomes abnormal and ultimately biliary system diseases occur. But the change in vivo is affected by multiple factors and is a multistage procedure. In vitro experiments can not absolutely reflect the conditions in vivo. So our experiment provides a clue to research the occurrence, development and treatment of biliary system diseases, but the certain role of bile duct fibroblasts and the certain mechanisms are still open to be elucidated.
Edited by Gupta MK and Xu FM
| 1. | Li XP, Ouyang KQ, Cai SX. The regulation of bile secretion and eduction. Shijie Huaren Xiaohua Zazhi. 2001;9:1066-1070. |
| 2. | Wei JG, Wang YC, Du F, Yu HJ. Dynamic and ultrastructural study of sphincter of Oddi in early-stage cholelithiasis in rabbits with hypercholesterolemia. World J Gastroenterol. 2000;6:102-106. [PubMed] |
| 3. | Lammert F, Südfeld S, Busch N, Matern S. Cholesterol crystal binding of biliary immunoglobulin A: visualization by fluorescence light microscopy. World J Gastroenterol. 2001;7:198-202. [PubMed] |
| 4. | Lui P, Chen DF. The separation and primary culture of canine gall-bladder epithelium. Shijie Huaren Xiaohua Zazhi. 2001;9:99-100. |
| 5. | Zapata R, Severín C, Manríquez M, Valdivieso V. Gallbladder motility and lithogenesis in obese patients during diet-induced weight loss. Dig Dis Sci. 2000;45:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Greaves RR, O'Donnell LJ, Farthing MJ. Differential effect of prostaglandins on gallstone-free and gallstone-containing human gallbladder. Dig Dis Sci. 2000;45:2376-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Gustafsson U, Sahlin S, Einarsson C. High level of deoxycholic acid in human bile does not promote cholesterol gallstone formation. World J Gastroenterol. 2003;9:1576-1579. [PubMed] |
| 9. | Bogdarin IuA, Kozlov DV. [Correction of lipid metabolism in rabbits with experimental cholelithiasis]. Vopr Med Khim. 2002;48:368-372. [PubMed] |
| 10. | Quallich LG, Stern MA, Rich M, Chey WD, Barnett JL, Elta GH. Bile duct crystals do not contribute to sphincter of Oddi dysfunction. Gastrointest Endosc. 2002;55:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Masclee AA, Vu MK. Gallbladder motility in inflammatory bowel diseases. Dig Liver Dis. 2003;35:S35-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Pallotta N. Ultrasonography in the assessment of gallbladder motor activity. Dig Liver Dis. 2003;35:S67-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Rhee JY, Elta GH. The relationship of bile duct crystals to sphincter of Oddi dysfunction. Curr Gastroenterol Rep. 2003;5:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Kohut M, Nowak A, Nowakowska-Duiawa E, Marek T. Presence and density of common bile duct microlithiasis in acute biliary pancreatitis. World J Gastroenterol. 2002;8:558-561. [PubMed] |
| 15. | Zanlungo S, Nervi F. The molecular and metabolic basis of biliary cholesterol secretion and gallstone disease. Front Biosci. 2003;8:s1166-s1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Toouli J. Biliary Dyskinesia. Curr Treat Options Gastroenterol. 2002;5:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wang XJ, Wei JG, Wang CM, Wang YC, Wu QZ, Xu JK, Yang XX. Effect of cholesterol liposomes on calcium mobilization in muscle cells from the rabbit sphincter of Oddi. World J Gastroenterol. 2002;8:144-149. [PubMed] |
| 18. | Wang XJ, Wei JG, Wang YC, Xu JK, Wu QZ, Wu DC, Yang XX. Effect of cholesterol liposome on contractility of rabbit Oddi's sphincter smooth muscle cells. Shijie Huaren Xiaohua Zazhi. 2000;8:633-637. |
| 19. | Corazziari E. Sphincter of Oddi dysfunction. Dig Liver Dis. 2003;35:S26-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Avisse C, Flament JB, Delattre JF. Ampulla of Vater. Anatomic, embryologic, and surgical aspects. Surg Clin North Am. 2000;80:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Wei JG, Wang YC, Liang GM, Wang W, Chen BY, Xu JK, Song LJ. The study between the dynamics and the X-ray anatomy and regularizing effect of gallbladder on bile duct sphincter of the dog. World J Gastroenterol. 2003;9:1014-1019. [PubMed] |
| 22. | Savrasov VM. [Functional radiographic anatomy of the terminal sphincter duct of the biliary pancreatic system]. Eksp Klin Gastroenterol. 2002;6:81-82. [PubMed] |
| 23. | Chen BY, Wei JG, Wang YC, Yang XX, Qian JX, Yu J, Chen ZN, Xu J, Wu DC. Effects of cholesterol on the proliferation of cul-tured rabbit bile duct fibroblasts. Shijie Huaren Xiaohua Zazhi. 2002;10:566-570. |
| 24. | Chen BY, Wei JG, Wang YC, Wang CM, Yu J, Yang XX. Effects of cholesterol on the phenotype of rabbit bile duct fibroblasts. World J Gastroenterol. 2003;9:351-355. [PubMed] |
| 25. | Laplante AF, Germain L, Auger FA, Moulin V. Mechanisms of wound reepithelialization: hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001;15:2377-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Jun JB, Kuechle M, Harlan JM, Elkon KB. Fibroblast and endothelial apoptosis in systemic sclerosis. Curr Opin Rheumatol. 2003;15:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1201] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 28. | Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S87-S92. [PubMed] |
| 29. | Tejero-Trujeque R. How do fibroblasts interact with the extracellular matrix in wound contraction. J Wound Care. 2001;10:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Bisson MA, McGrouther DA, Mudera V, Grobbelaar AO. The different characteristics of Dupuytren's disease fibroblasts derived from either nodule or cord: expression of alpha-smooth muscle actin and the response to stimulation by TGF-beta1. J Hand Surg Br. 2003;28:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Piper C, Schultheiss HP, Akdemir D, Rudolf J, Horstkotte D, Pauschinger M. Remodeling of the cardiac extracellular matrix differs between volume- and pressure-overloaded ventricles and is specific for each heart valve lesion. J Heart Valve Dis. 2003;12:592-600. [PubMed] |
| 33. | Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp Physiol. 2002;87:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 385] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 36. | Papakonstantinou E, Aletras AJ, Roth M, Tamm M, Karakiulakis G. Hypoxia modulates the effects of transforming growth factor-beta isoforms on matrix-formation by primary human lung fibroblasts. Cytokine. 2003;24:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Das M, Dempsey EC, Reeves JT, Stenmark KR. Selective expansion of fibroblast subpopulations from pulmonary artery adventitia in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L976-L986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Bogatkevich GS, Tourkina E, Abrams CS, Harley RA, Silver RM, Ludwicka-Bradley A. Contractile activity and smooth muscle alpha-actin organization in thrombin-induced human lung myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;285:L334-L343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Eyden B. Electron microscopy in the study of myofibroblastic lesions. Semin Diagn Pathol. 2003;20:13-24. [PubMed] |
| 40. | Ehrlich HP, Diez T. Role for gap junctional intercellular communications in wound repair. Wound Repair Regen. 2003;11:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Li XP, Mao XZ. Effect of estrogen, cholic acid loading and bile draining on hepatobiliary functions in rats. Shijie Huaren Xiaohua Zazhi. 2000;8:1009-1012. |
| 42. | Zhu XF, Chen GH, He XS, Lu MQ, Wang GD, Cai CJ, Yang Y, Huang JF. Liver transplantation and artificial liver support in fulminant hepatic failure. World J Gastroenterol. 2001;7:566-568. [PubMed] |
| 43. | He XS, Huang JF, Liang LJ, Lu MD, Cao XH. Surgical resection for hepatoportal bile duct cancer. World J Gastroenterol. 1999;5:128-131. [PubMed] |
| 44. | Yang HM, Wu J, Li JY, Zhou JL, He LJ, Xu XF. Optic properties of bile liquid crystals in human body. World J Gastroenterol. 2000;6:248-251. [PubMed] |
| 45. | Diana A, Pietra M, Guglielmini C, Boari A, Bettini G, Cipone M. Ultrasonographic and pathologic features of intestinal smooth muscle hypertrophy in four cats. Vet Radiol Ultrasound. 2003;44:566-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Cheng AC, Wang MS, Chen XY, Guo YF, Liu ZY, Fang PF. Pathogenic and pathological characteristic of new type gosling viral enteritis first observed in China. World J Gastroenterol. 2001;7:678-684. [PubMed] |
| 47. | Bettini G, Muracchini M, Della Salda L, Preziosi R, Morini M, Guglielmini C, Sanguinetti V, Marcato PS. Hypertrophy of intestinal smooth muscle in cats. Res Vet Sci. 2003;75:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Gunst SJ, Tang DD, Opazo Saez A. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol. 2003;137:151-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Schelling JR, Sinha S, Konieczkowski M, Sedor JR. Myofibroblast differentiation: plasma membrane microdomains and cell phenotype. Exp Nephrol. 2002;10:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Prajapati DN, Hogan WJ. Sphincter of Oddi dysfunction and other functional biliary disorders: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:601-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |