Published online Mar 15, 2004. doi: 10.3748/wjg.v10.i6.837
Revised: August 23, 2003
Accepted: September 18, 2003
Published online: March 15, 2004
AIM: To observe the adsorbent effect of resin on endotoxin, cytokine, bilirubin in plasma of patients with hepatic failure and to determine the resin perfusion as an artificial liver support system in the treatment of hepatic failure.
METHODS: One thousand milliliters of discarded plasma was collected from each of 6 severe hepatitis patients treated with plasma exchange. The plasma was passed through a resin perfusion equipment for 1-2 h via extracorporeal circulation, and then absorbent indicators of transaminase, bilirubin, blood ammonia, endotoxin and cytokines were examined. In the meantime, study of in vivo resin plasma perfusion was performed on 7 severe hepatitis patients to compare the changes of endotoxin and cytokines in blood before and after perfusion.
RESULTS: The levels of total bilirubin, endotoxin, interleukin 1β and TNF-α in plasma were significantly decreased after in vitro resin plasma perfusion. The levels of interleukin 1β, TNF-α and endotoxin in blood were also evidently declined after in vivo resin plasma perfusion. Nevertheless, no obvious changes in IL-6, creatinine (Cr) and urea nitrogen (UN), blood ammonia and electrolytes were found both in vitro and in vivo.
CONCLUSION: Bilirubin, endotoxin and cytokines in plasma of patients with hepatic failure can be effectively adsorbed by resin in vitro. Most cytokines and endotoxin in plasma can also be effectively removed by resin in vivo. It demonstrates that resin perfusion may have good treatment efficacy on hepatic failure and can be expected to slow down the progression of hepatic failure.
- Citation: Wang YJ, Wang ZW, Luo BW, Liu HL, Wen HW. Assessment of resin perfusion in hepatic failure in vitro and in vivo. World J Gastroenterol 2004; 10(6): 837-840
- URL: https://www.wjgnet.com/1007-9327/full/v10/i6/837.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i6.837
In the research field of artificial liver, blood perfusion, blood filtration, plasma exchange techniques and methods are called non-biological artificial liver[1-3]. They are based on the mechanical mechanism of blood purification to remove toxins in body to achieve the treatment goal for hepatic failure[4-11]. New-style resin blood perfusion is mostly used in toxicosis rescue and uremia, etc in clinic, but was rarely reported in the treatment of hepatic failure. Since endotoxin and cytokine play a pivotal role in the pathogenesis of hepatic failure in severe hepatitis[12-16], it has become an important issue whether resin blood perfusion can effectively remove endotoxin and cytokine in body. Therefore, we examined the effect of a non-bioartificial liver with resin plasma perfusion on the plasma endotoxin and cytokine removal in patients with severe hepatitis to determine and assess the curative effect of resin plasma perfusion in the treatment of severe hepatitis.
Thirteen subjects with severe viral hepatitis (female 1, male 12, aged 28 to 57 years with a mean of 41.3 years) were enrolled in this study. All subjects belonged to chronic severe hepatitis caused by HBV infection according to the diagnostic criteria described in the Viral Hepatitis Protection and Cure Guideline established on a national conference. Of these patients, 8 were in middle stage, 5 in final stage, complication of encephalopathy, spontaneous peritonitis and hepatorenal syndrome was found in 11, 5 and 3, respectively.
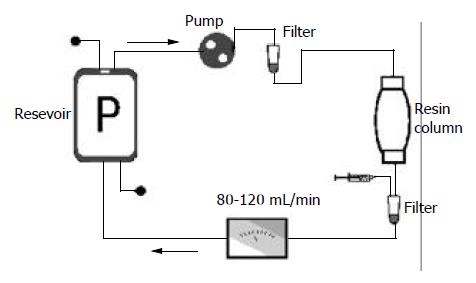
One thousand milliliters of discarded plasma was collected from each of 6 severe hepatitis patients treated with plasma exchange, and mimic blood perfusion was performed in 3 h. The resin perfusion device (Lizhu Bio-material Company, Zhuhai, China) and tubes were filled with 500 mL of 50 g/L glucose injection, then rinsed with 3 000 mL of normal saline containing 80 mg heparin from bottom to top at 50-100 mL/min. In the mean time, perfusion device was tapped by hand and rotated to exclude bubbles and particles. Plasma perfusion was performed for 1-2 h (average 1.7 h) and the velocity of plasma cycling through the perfusion device was at 80-120 ml/min (Figure 1). Plasma before and after circulation was collected, sealed and stored at -40°C. All the procedures, such as plasma collection, perfusion in vitro and sample harvesting used aseptic technique, syringe, cycling tubes and frozen tubes were radiated by 60Co to remove pyrogen.
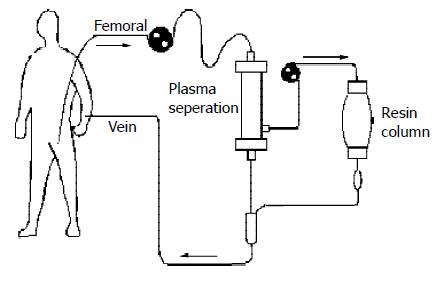
Resin plasma perfusion was performed in 7 patients with severe hepatitis. Temporal artery-vein circuit was established by insertion of femoral and pectoral venous cannulas. The patients were heparinized based on individual conditions. The first dose was 1-1.5 mg/kg, then 0.1 mg/kg each 30 min was added. The patients’ blood was introduced to a plasma separator (Fresenius Medical Care, Bad Homblurg, GER) at 100-120 mL/min for separation of plasma from the whole blood. The separated plasma was absorbed by the resin perfusion device, then mixed with separated blood cells, and returned to patients. The perfusion was maintained for 1-2 h (average 1.6 h) (Figure 2). The plasma before and after perfusion was collected and stored as the above methods.
Plasma aminotransferase, total bilirubin (TB), total bile acid (TBA), electrolyte, UN and Cr were measured using a biochemical autoanalyzer (Hitach Co., Tokyo, Japan). Blood ammonia was measured by a blood ammonia detector (Shiga Co., Tokyo, Japan). Endotoxin was measured by using a limulus test kit according to the manufacturer’s instructions (Yihua Medical Technology Co., Shanghai, China). The level of IL-1β and TNF-α was measured by using a radio immunoassay (RIA) kit according to manufacturer’s instructions (Department of Radio Immunoassay, PLA General Hospital Technical Development Center ). IL-6 level was measured by using an ELISA kit according to manufacturer’s instructions (Shengxong Medical Technology Co., Shanghai, China).
All results were expressed as mean ± SD. Comparisons between the groups and the same group before and after perfusion were analyzed by Student’s t test.
After resin perfusion device was used to absorb plasma of the patients with severe hepatitis in vitro, transaminase, TB and TBA were all declined with a statistically significant difference in TB, DB and IB before and after resin perfusion (t value was 2.081, 2.048, and 2.086 respectively, P < 0.05) (Table 1).
| Before absorption | After absorption | |
| ALT(IU/L) | 74.17 ± 49.68 | 47.83 ± 22.33 |
| AST(IU/L) | 88.83 ± 45.58 | 58.33 ± 44.64 |
| TB(mol/L) | 518.8 ± 180.27 | 356.13 ± 162.87a |
| TBA(mol/L) | 157.17 ± 53.53 | 131.67 ± 34.95 |
TBA was markedly declined in patients with in vivo resin plasma perfusion with a significant difference (P < 0.01). Nevertheless, transaminase and TB had no marked change before and after absorption (Table 2).
The electrolytes – K, Na, Cl, Ca, Mg, and P after absorption had no significant changes compared to those before absorption. UN and Cr were declined after absorption with no statistical significance compared to those before absorption with UN value of 6.79 ± 4.14 mmol/L before absorption and 6.42 ± 3.39 mmol/L after absorption, and Cr value of 135.67 ± 30.77 μmol/L before absorption and 121.85 ± 20.40 μmol/L after absorption.
After in vitro resin absorption, blood ammonia in plasma was not markedly decreased (98.17 ± 20.60 μmol/L and 91.50 ± 18.84 μmol/L). Similar to the result of in vitro, blood ammonia had no significant difference before and after in vivo resin plasma perfusion (48.25 ± 8.63 μmol/L and 46.75 ± 9.44 μmol/L).
The level of LPS was markedly decreased after in vitro absorption (t = 6.604, P < 0.01). The changes in TNF-α and IL-1β had a statistical significance (t was 2.876 and 3.673 respectively, P < 0.05). IL-6 had no significant change before and after in vitro resin absorption (Table 3).
The plasma endotoxin was markedly decreased after in vivo resin plasma perfusion (t = 5.233, P < 0.001). TNF-α and IL-1β also changed significantly (t value was 3.474 and 4.869 respectively, P < 0.01) with declined levels of 16.3% and 37.1% respectively. IL-6 had no evident change before and after in vivo plasma perfusion (Table 4).
Blood perfusion is a commonly used method for blood purification[17-20]. Activated charcoal representing perfusion equipment is most widely applied in the clinical treatment for drug toxicosis, uremia and liver coma and a good result has been obtained[21,22]. However, the shape of activated charcoal particles is irregular and their mechanical strength is inferior, thus particles are easily broken. If a broken particle directly contacts with blood, it will cause hemolysis and microvessel occlusion. Therefore, activated charcoal must be encapsulated before use. Synthetic resin is another kind of medical absorbent material with macropore high molecular polymers belonging to neutral macropore resin that absorbs substances via Vandar Val gravitation. Its absorbent ability has been found to be characterized by a fast absorbent speed, high mechanical strength, relative absorbent specificity mainly absorbing the substances with a molecular weight of 500-5 000 Da and showing an outstanding absorbent ability to those toxins which can bind to proteins closely or are highly fat-soluble[23,24].
In the experiment of resin blood perfusion in vitro, we found that the resin blood perfusion device was able to effectively absorb transaminase and bilirubin but weakly affect kidney function and blood ammonia, considering that it absorbed medium molecules not small molecules. It illustrated the resin blood perfusion could improve the hepatic function in patients with severe hepatitis. In the meantime, it did not cause electrolyte imbalance and disturbance in the internal environment. Therefore, resin blood perfusion can be used as an artificial liver support system in the treatment of severe hepatitis.
Current studies considered that the pathogenesis of severe hepatitis was the superposition of virus-caused primary immunopathological damage and cytokine-induced secondary damage[25-27]. When liver barrier function was impaired, endotoxaemia would occur. Endotoxins stimulated the mononuclear phagocyte system inside and outside the liver, and thus, enormous cytokines were released. Furthermore, this cytokine-induced secondary hepatocellular damage played an important role in the course of hepatitis. Hereby, cytokine removal was good for alleviating liver damage, reducing leukoplania and thrombocyte aggregates, maintaining internal environmental equilibrium, and sequentially slowing down or even reversing the progression of disease and improving prognosis[28-30].
Along with the development in molecular biology, research of antagonists for various cytokines has been launched, for example, monoclonal antibody, receptor antibody, soluble antibody. However, their clinical application is not ideal. In recent years, more and more researchers have began to use blood purification to remove cytokines[31-33]. At present, it is still disputable whether blood purification can effectively remove cytokines[34,35]. Some researchers held that leucocytes could be activated by passing through the device during blood perfusion to cause the releasing of cytokines. In addition, it has been found the molecular weight of cytokines is relatively high, and cytokines are mostly bound to proteins in plasma, the half-life of cytokines is short and they are produced and metabolized quickly. Therefore, it would be hard to remove them via blood purification[36].
In this study, after plasma perfusion treatment for severe hepatitis, both TNF-α and IL-1β in plasma were significantly decreased, illustrating that plasma perfusion was an effective method for cytokine removal. The change of IL-6 was not significant, because IL-6 might be produced too fast, or it related to different absorbent efficacy of the perfusion device to various cytokines.
In conclusion, resin can effectively absorb bilirubin, LPS, TNF-α and IL-1βin vitro, and LPS, TNF-α and IL-1β can be significantly decreased in resin plasma perfusion in vivo, resin perfusion has good curative effects on severe hepatitis with hepatic failure.
Edited by Zhang JZ and Wang XL Proofread by Xu FM
| 1. | Dowling DJ, Mutimer DJ. Artificial liver support in acute liver failure. Eur J Gastroenterol Hepatol. 1999;11:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 3. | Stockmann HB, Hiemstra CA, Marquet RL, IJzermans JN. Extracorporeal perfusion for the treatment of acute liver failure. Ann Surg. 2000;231:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Borra M, Galavotti D, Bellini C, Fumi L, Morsiani E, Bellini G. Advanced technology for extracorporeal liver support system devices. Int J Artif Organs. 2002;25:939-949. [PubMed] |
| 5. | Bertani H, Gelmini R, Del Buono MG, De Maria N, Girardis M, Solfrini V, Villa E. Literature overview on artificial liver support in fulminant hepatic failure: a methodological approach. Int J Artif Organs. 2002;25:903-910. [PubMed] |
| 6. | Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamura M. Continuous hemofiltration/hemodiafiltration in critical care. Ther Apher. 2002;6:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Ash SR, Caldwell CA, Singer GG, Lowell JA, Howard TK, Rustgi VK. Treatment of acetaminophen-induced hepatitis and fulminant hepatic failure with extracorporeal sorbent-based devices. Adv Ren Replace Ther. 2002;9:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Clemmesen JO, Kondrup J, Nielsen LB, Larsen FS, Ott P. Effects of high-volume plasmapheresis on ammonia, urea, and amino acids in patients with acute liver failure. Am J Gastroenterol. 2001;96:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Steczko J, Bax KC, Ash SR. Effect of hemodiabsorption and sorbent-based pheresis on amino acid levels in hepatic failure. Int J Artif Organs. 2000;23:375-388. [PubMed] |
| 10. | Hughes RD. Review of methods to remove protein-bound substances in liver failure. Int J Artif Organs. 2002;25:911-917. [PubMed] |
| 11. | Di Campli C, Zileri Dal Verme L, Andrisani MC, Armuzzi A, Candelli M, Gaspari R, Gasbarrini A. Advances in extracorporeal detoxification by MARS dialysis in patients with liver failure. Curr Med Chem. 2003;10:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci. 2001;46:1700-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Galun E, Axelrod JH. The role of cytokines in liver failure and regeneration: potential new molecular therapies. Biochim Biophys Acta. 2002;1592:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Streetz K, Leifeld L, Grundmann D, Ramakers J, Eckert K, Spengler U, Brenner D, Manns M, Trautwein C. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology. 2000;119:446-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, Yumoto Y, Tanimoto T, Kurimoto M, Tanaka N. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961-965. [PubMed] |
| 17. | Sarnatskaya VV, Lindup WE, Walther P, Maslenny VN, Yushko LA, Sidorenko AS, Nikolaev AV, Nikolaev VG. Albumin, bilirubin, and activated carbon: new edges of an old triangle. Artif Cells Blood Substit Immobil Biotechnol. 2002;30:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Nakamura T, Ushiyama C, Suzuki Y, Inoue T, Shoji H, Shimada N, Koide H. Combination therapy with polymyxin B-immobilized fibre haemoperfusion and teicoplanin for sepsis due to methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2003;53:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Tsuchida K, Takemoto Y, Sugimura K, Yoshimura R, Nakatani T. Direct hemoperfusion by using Lixelle column for the treatment of systemic inflammatory response syndrome. Int J Mol Med. 2002;10:485-488. [PubMed] |
| 20. | Sechser A, Osorio J, Freise C, Osorio RW. Artificial liver support devices for fulminant liver failure. Clin Liver Dis. 2001;5:415-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Ash SR. Extracorporeal blood detoxification by sorbents in treatment of hepatic encephalopathy. Adv Ren Replace Ther. 2002;9:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Kramer L, Gendo A, Madl C, Ferrara I, Funk G, Schenk P, Sunder-Plassmann G, Hörl WH. Biocompatibility of a cuprophane charcoal-based detoxification device in cirrhotic patients with hepatic encephalopathy. Am J Kidney Dis. 2000;36:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Musanje L, Darvell BW. Polymerization of resin composite restorative materials: exposure reciprocity. Dent Mater. 2003;19:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Gode F, Pehlivan E. A comparative study of two chelating ion-exchange resins for the removal of chromium(III) from aqueous solution. J Hazard Mater. 2003;100:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Endo Y, Shibazaki M, Yamaguchi K, Kai K, Sugawara S, Takada H, Kikuchi H, Kumagai K. Enhancement by galactosamine of lipopolysaccharide(LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment. Br J Pharmacol. 1999;128:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Masai T, Sawa Y, Ohtake S, Nishida T, Nishimura M, Fukushima N, Yamaguchi T, Matsuda H. Hepatic dysfunction after left ventricular mechanical assist in patients with end-stage heart failure: role of inflammatory response and hepatic microcirculation. Ann Thorac Surg. 2002;73:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Rahman T, Hodgson H. Clinical management of acute hepatic failure. Intensive Care Med. 2001;27:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Stange J, Mitzner SR, Klammt S, Freytag J, Peszynski P, Loock J, Hickstein H, Korten G, Schmidt R, Hentschel J. Liver support by extracorporeal blood purification: a clinical observation. Liver Transpl. 2000;6:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Stange J, Mitzner SR, Risler T, Erley CM, Lauchart W, Goehl H, Klammt S, Peszynski P, Freytag J, Hickstein H. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs. 1999;23:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Nakae H, Yonekawa C, Wada H, Asanuma Y, Sato T, Tanaka H. Effectiveness of combining plasma exchange and continuous hemodiafiltration (combined modality therapy in a parallel circuit) in the treatment of patients with acute hepatic failure. Ther Apher. 2001;5:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (5)] |
| 31. | Nakamura T, Ushiyama C, Suzuki S, Shimada N, Ebihara I, Suzaki M, Takahashi T, Koide H. Effect of plasma exchange on serum tissue inhibitor of metalloproteinase 1 and cytokine concentrations in patients with fulminant hepatitis. Blood Purif. 2000;18:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Nakae H, Asanuma Y, Tajimi K. Cytokine removal by plasma exchange with continuous hemodiafiltration in critically ill patients. Ther Apher. 2002;6:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 33. | Ho DW, Fan ST, To J, Woo YH, Zhang Z, Lau C, Wong J. Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model. Gut. 2002;50:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Ryan CJ, Anilkumar T, Ben-Hamida AJ, Khorsandi SE, Aslam M, Pusey CD, Gaylor JD, Courtney JM. Multisorbent plasma perfusion in fulminant hepatic failure: effects of duration and frequency of treatment in rats with grade III hepatic coma. Artif Organs. 2001;25:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Nakae H, Yonekawa T, Narita K, Endo S. Are proinflammatory cytokine concentrations reduced by plasma exchange in patients with severe acute hepatic failure. Res Commun Mol Pathol Pharmacol. 2001;109:65-72. [PubMed] |
| 36. | Iwai H, Nagaki M, Naito T, Ishiki Y, Murakami N, Sugihara J, Muto Y, Moriwaki H. Removal of endotoxin and cytokines by plasma exchange in patients with acute hepatic failure. Crit Care Med. 1998;26:873-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |