INTRODUCTION
The antiproliferative action of somatostatin (SS)/analogues is signaled by specific G-protein coupled receptors, and up to date, six different subtypes (SSTR-1, -2A, -2B, -3, -4, and -5) have been cloned and functionally characterized in various cell systems, including pancreas, adrenal cortex, and brain tissue[1]. However, several studies demonstrated that somatostatin receptor subtypes (SSTRs) were strongly expressed in the normal pancreas, whereas not only a desensitization or mutation of these receptors occurred in pancreatic tumors[2], but also expression of these receptors, especially SSTR2, was frequently lost in human pancreatic adenocarcinomas[3-5]. Despite remarkable biochemical properties of SS analogues in vitro, poor therapeutic results with them in Phase I/II clinical trials against the majority of cases of pancreatic cancers[5-7], were due to the loss of gene expression for SSTR2 in pancreatic cancers and relatively low expressions of SSTR3 and SSTR5[8].
VEGF, also known as a vascular permeability factor, is highly expressed in various types of tumors, and is an endothelial cell (EC) specific mitogen. VEGF via binding to its high affinity receptors (Flt-1/VEGFR-1, Flk-1/KDR/VEGFR-2), exerts its mitogenic effect by promoting EC proliferation and migration, thereby playing a crucial role in tumor neovascularization[9,10]. Gupta et al[11] reported VEGF as a survival factor for EC, rendering these cells more radioresistant. Several studies showed positive correlations between tumor cell VEGF expression, blood vessel density, tumor growth and metastasis, disease progression and poor prognosis in pancreatic carcinomas[12-14], and suppression of VEGF expression attenuated pancreatic cancer cell tumorigenicity[10], suggesting that over-expression of VEGF might be associated with the aggressive phenotype of this disease and that VEGF should be an important target for anticancer therapy.
On the other hand, matrix metalloproteinases (MMPs) were reported to enhance the degradation of extracellular matrix (ECM), and thereby hydrolyze the important components of ECM and basement membrane such as types IV, V, VII, X collagens and fibronectin, elastin, etc and were closely associated with the invasiveness and metastasis of tumors[15-20]. MMP-2 was the most commonly expressed MMPs in pancreatic tumor specimens but not in normal pancreas and was correlated with the aggressive phenotype (invasive and metastatic potential) of pancreatic carcinomas[21-24]. Ellenrieder et al[25] demonstrated that increased expression and activation levels of MMP-2 were strongly associated with elevated expression levels of its activators MT1-MMP (Membrane type 1-MMP) and MT2-MMP (Membrane type 2-MMP) and that it played a significant role in pancreatic tumor cell invasion. The selective MMP-2 and MMP-9 inhibitor MMI-166 exerted its antitumor effects on pancreatic cancer by inhibiting its invasion and angiogenesis[26].
In the present study, we evaluated the potency of introduction of exogenous SSTR2 gene as negative regulators of VEGF and MMP-2 production in pancreatic cancer cell line PC-3 for the following reasons: (1) SSTR2 was strongly expressed in normal pancreas whereas it was frequently lost in human pancreatic adenocarcinoma[2-4]. (2) Antitumor effects of SSTR2 occurred as a consequence of an SSTR2-dependent negative feedback autocrine loop, whereby SSTR2 induced the expression of its own ligand, SS, which also constitutively activated SSTR2[4,27]. (3) SS/analogues exhibited antisecretory effects by the inhibition of release of growth factors and trophic hormones, e.g., growth hormone, insulin-like growth factor-1, insulin, gastrin[2]. In addition, since pancreatic cancer is characterized by the over-expression of several angiogenic factors such as VEGF, bFGF, MMP-2, etc, it is necessary to elucidate the effects of the genes or molecules with antiangiogenic properties on pancreatic cancer. Previous studies have reported the antiangiogenic properties of SS/analogues and SSTRs in various tumors[28,29], however the mechanisms involved in the antiangiogenic effects of SSTR2 have been poorly elucidated in this pancreatic cancer cell line PC-3 as yet. Hence, our present study demonstrated that the reexpression of SSTR2 gene in the pancreatic cancer cell line PC-3 devoid of SSTR2, exhibited its antiangiogenic effects by down-regulating the expression of angiogenic factors in vitro both at protein and mRNA levels.
MATERIALS AND METHODS
Materials
PC-3, a human pancreatic cancer cell line, was obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Dulbecco’s modified eagles medium (DMEM), fetal bovine serum (FBS), lipofectamine and geneticin (G418) were purchaesd from Gibco BRL. The full-lenth cDNA of human SSTR2 was kindly provided by G. I. Bell (Howard Hughes Medical Institute, Chicago, IL). Monoclonal antibody of SSTR2, mouse anti-VEGF polyclonal antibody, mouse anti-MMP-2 monoclonal antibody, SABC kit were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). TRIZOL® reagent, RNasin, oligo(dt) 15, dNTPs, M-MLV reverse transcriptase, Taq DNA polymerase and VEGF ELISA kit were from Promega. Eukaryotic expression vector pcDNA3.1 was purchased from Invitrogen (Invitrogen, San Diego, CA).
Plasmid construction and gene transfer
Human SSTR2 cDNA was digested with EcoRI/XbaI and cloned in the EcoRI/XbaI site of pcDNA3.1. The sequence encoding the signal peptide from alkaline phosphatase was inserted into the HindIII/EcoRI site upstream of SSTR2 and an epitope tag derived from influenza virus hemagglutinin A (HA) was fused in the EcoRI site between the signal peptide and SSTR2. Recombinant plasmid was purified by QIA prep spin miniprep kit (QIAGEN Co).
PC-3 cells were routinely cultured in DMEM media supplemented with 10% heat-inactivated FBS, 100 u/ml penicillin and 100 u/ml streptomycin, and incubated at 37 °C in a humidified atmosphere containing 5% CO2 in air. Gene transfer was performed according to the manufacture’s protocol of lipofectamine (Gibco BRL). Briefly, about 3 × 105 cells per well containing 2 ml appropriate complete growth medium were seeded in a six-well culture plate, and incubated at 37 °C in a CO2 incubator until the cells were 70% to 80% confluent. A cover slip was plated in each well before seeding. After the cells were ringed with serum free and antibiotics-free medium, the cells were transfected separately with pcDNA3.1-SSTR2 1 μg/lipofectamine 3 μL (experimental group), pcDNA3.1 1 μg/lipofectamine 3 μL (vector control), and only lipofectamine 3 μL (mock control), followed by incubation at 37 °C in a CO2 incubator for 6 hours. Then the medium was replaced by DMEM culture medium containing 20% FBS. After 48 hours, two wells in each group were taken out to detect the transient expression of SSTR2 by immunohistochemical SABC methods, whereas others were continuously cultured for stable expression of SSTR2. G418 (500 μg/L) was added to select the resistant clones after 48 hours. Six days later, when most of the cells died, the concentration of G418 was decreased to 300 μg/L and cells were cultured with G418 for another 6 days. Then the medium was changed every 3 or 4 days and colonies were collected approximately 2 weeks later for the examination of stable expression of SSTR2 by immunohistochemical SABC methods and RT-PCR assay.
Confirmation of SSTR2 protein expression by immunohistochemical staining
The stable expression of SSTR2 in the experimental group cells was detected by using immunohistochemical SABC methods. All the cover slips were dried at room temperature and washed twice with PBS solution (pH7.2), followed by the treatment with 3% H2O2 for 10 min at room temperature. Then the cover slips were incubated in 5% bovine serum albumin in PBS solution for 20 min to block the nonspecific antibody-binding. The cover slips were then incubated with mouse anti-human SSTR2 antibody, diluted in 1:50 in 0.5% bovine serum albumin in PBS for 12 hours at 4 °C. The bridging antibody (biotinylated goat anti-mouse IgG) and SABC complex were diluted to 1:100 and incubated with the specimens for 20 min at 30 °C. Finally diaminobenzidine tetrachloride (DAB) was used for color development and the cover slips were counterstained with hematoxylin. Positive rate (brown color cells) was automatically measured with the biological image analysis system 2000 (Opton Germany).
Confirmation of SSTR2 mRNA expression by RT-PCR
Total RNA was extracted separately from PC-3 cells of each group with TRIZOL® reagent following the manufacturer’s instructions. A 2 μg (treated in 5 μL DEPC water in an Ep tube) sample of total RNA was denaturalized by incubating at 70 °C for 5 min, and the tube was placed to ice for 3 min, and then reverse-transcribed into complementary DNA (cDNA) by using Moloney murine leukemia virus reverse transcriptase (M-MLV). Briefly, the denaturalized RNA (5 μL) was incubated for 60 min at 37 °C and for 5 min at 95 °C with 4 μL 5 × reverse transcriptase buffer, 1 μL oligo (dt) 15, 1 μL RNasin (50 u/mL), 1 μL dNTPs (10 mmol/L), 1 μL reverse transcriptase (200 u/mL), and 7 μL DEPC water in a total volume of 20 μL. For polymerase chain reaction (PCR), 5 μL of the resulting cDNA, 31 μL of tripled-distilled H2O, 5 μL of 10 × PCR buffers, 3 μL of μgCl2 (25 mmol/L), 1 μL of dNTPs, 1 μL of each of sense and antisense primers (10 pmol/L), 1 μL of each of sense and antisense β-actin, and 1 μL Taq DNA polymerase (3 u/mL) in a total volume of 50 μL were added. The samples were amplified through 35 cycles, each amplification consisting of denaturation at 94 °C for 40 s, primer annealing at 55 °C for 40 s and extension at 72 °C for 1 min. Cycles were preceded by incubation at 94 °C for 5 min to ensure full denaturation of the target gene, followed by an extra incubation at 72 °C for 10 min to ensure full extentsion of the products. PCR products were analyzed on 1.5% agarose gel containing ethidium bromide. The sequences of the primers for SSTR2 were sense 5’-CCCCAGCCCTTAAAGGCATGT-3’ and antisense 5’-GGTCTCCATTGAGGAGGGTCC-3’ (234 bp) and for β-actin, sense 5’-GTGCGTGACATTAAGGAG-3’ and antisense 5’-CTAAGTCATAGTCCGCCT-3’ (520 bp).
Detection of VEGF mRNA and MMP-2 mRNA expression by RT-PCR
Total RNA was extracted separately from PC-3 cells of each group with TRIZOL® reagent following the manufacturer’s instructions. RT-PCR was carried out as described above except some changes in conditions of amplification cycles. For mRNA expression of VEGF, samples of each group were subjected to PCR at an annealing temperature from 60 °C to 50 °C decreased by 0.5 °C per cycle for 20 cycles, followed by an additional 15 cycles at an annealing temperature of 50 °C for 35 s. PCR products were analyzed on 1.5% agarose gel containing ethidium bromide and quantified by a complete gel documentation and analysis system. VEGF mRNA expression level was determined by the ratio of VEGF/β-actin protein. The sequences of primers for VEGF were sense 5’-TTGCTGCTCTACCTCCAC-3’ and antisense 5’-CTCCAGGCCCTCGTCATT-3’ (240 bp) and for β-actin, sense 5’-GTGCGTGACATTAAGGAG-3’ and antisense 5’-CTAAGTCATAGTCCGCCT-3’ (520 bp).
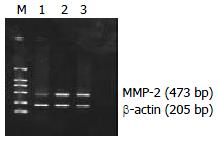
For mRNA expression of MMP-2, samples of each group were subjected to PCR for 33 cycles, each cycle consisting of denaturation at 94 °C for 1 min, primer annealing at 55 °C for 35 s and extentsion at 72 °C for 1 min. PCR products were analyzed on 1.5% agarose gel containing ethidium bromide and quantified by a complete gel documentation and analysis system. MMP-2 mRNA expression level was determined by the ratio of MMP-2/β-actin protein. The sequences of primers for MMP-2 were sense 5’-GCGGATCCAGCGCCCAGAGAGACAC-3’ and antisense 5’-TTAAGCTTCCACTCCGGGCAGGATT-3’ (473 bp) and for β-actin were sense 5’-CCTTCCTGGGCAT-GGAGTCCTG-3’ and antisense 5’-GGAGCAATGATCTTG-ATCTTC-3’ (205 bp)
Determination of VEGF concentration in cultured supernatants by ELISA
After successful stable transfection, VEGF protein levels in the supernatants secreted by the cultured human pancreatic cancer cells of experimental group, vector control and mock control were quantitated by ELISA. To generate the conditioned medium, the cells of each group (4 × 105/well) were incubated in 1.5 ml DMEM for 48 hours. The conditioned medium was then collected and centrifuged at 12000 rpm at 4 °C for 15 min, and then ELISA analysis was performed according to the manufacturer’s instructions. Furthermore, to observe the long-term antisecretory effect of SSTR2, ELISA analysis was performed two months after stable transfection. The value of OD (A450 values) of each well was measured at 450 nm. The supernatants were harvested in triplicate and the experiment was performed twice.
Detection of VEGF and MMP-2 expressions in vitro by immunohistochemistry
Two cover slips were plated in each well of six-well culture plates, and then the cells of each group (105 cells) containing 2 ml appropriate complete growth medium were seeded in each well, and incubated at 37 °C in a CO2 incubator for 48 hours. All the cover slips were dried and washed three times with PBS (pH7.2) at room temperature, followed by the treatment with the 1:1 mixture of 100% acetone and formaldehyde for 10 min at room temperatue. The expressions of VEGF and MMP-2 were detected by using immunohistochemical SABC methods as described above by using mouse anti-human VEGF polyclonal antibody and MMP-2 monoclonal antibody. Under the light microscope, positive staining (brown yellow) was located in cytoplasm and membrane for VEGF and MMP-2. For the image analysis, 150 cells of clear outline from 10 microscopic fields (15 cells in each field) were selected randomly from each group under 10 × 40 magnification, and the average gray value and integral optical density of each group were automatically measured by using HPIAS-1000, high resolution pathological image analysis.
Statistical analysis
Results were expressed as mean ± SD and the mean values were compared by using the ANOVA (SNK, Student-Newman-Keuls test) in the SAS 8.1 software and P < 0.05 was considered statistically significant.
RESULTS
Reexpression of SSTR2 after transfection
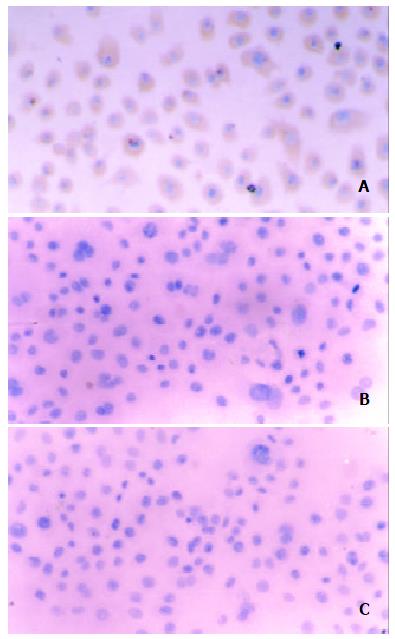
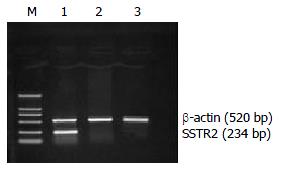
After 48 h in vitro transfection, most of the cells in experimental group demonstrated positive staining for SSTR2 as detected by immunohistochemical SABC methods, whereas almost no positive SSTR2 stainings were detected in vector control and mock control. The cells in experimental group were continuously cultured by adding G418 (500 mg/L) with 20% FBS, and then we were able to select a population of PC-3 cells resistant to the toxic effects of G418. After 2 weeks, all of these cells demonstrated positive staining for SSTR2 detected by immun o histo ch emical SABC meth od s (Figure 1) . Furthermore, RT-PCR analysis of total RNA extracted from the cells of each group showed SSTR2 mRNA expression in experimental group, but not in vector control and mock control (Figure 2). The results suggested that the exogenous SSTR2 gene was successfully reexpressed in PC-3 cell line devoid of SSTR2.
Figure 1 Immunohistochemical staining of SSTR2 expression after stable transfection The cytoplasmic brown yellow staining represents SSTR2 expression.
(A) experimental group, (B) vector control, and (C) mock control (SABC × 150).
Figure 2 Expression of SSTR2 mRNA by RT-PCR analysis in experimental group, but not in vector control and mock control.
Lane M: DNA marker DL 2000, Lane 1: experimental group, Lane 2: vector control, Lane 3: mock control.
Effect of SSTR2 on VEGF production in cultured supernatants
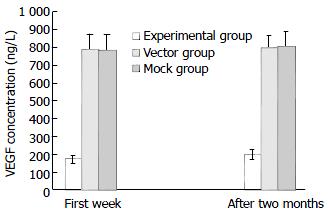
In the first week after stable transfection, VEGF levels in the cultured supernatants were significantly decreased in the cells of experimental group (172.63 ± 21.2 ng/L) compared with those of vector control (790.39 ± 86.52 ng/L) and mock control (786.42 ± 90.62 ng/L) (P < 0.05, Figure 3). Similarly, two months after stable transfection, there was still a significant inhibition of VEGF production in the cells of experimental group (198.85 ± 26.44 ng/L) compared with those of vector control (795.69 ± 72.35 ng/L) and mock control (805.32 ± 84.36 ng/L) (P < 0.05, Figure 3), however the inhibition of VEGF production in the cells of experimental group was slightly less than that in the first week (Figure 3). There were no statistical difference in VEGF levels between the vector control and mock control (Figure 3). Thus, SSTR2 could suppress the production of VEGF secreted by the cells of experimental group.
Figure 3 Inhibition of VEGF production in cultured superna-tants by SSTR2.
VEGF was determined in cultured superna-tants by ELISA in the first week and two months after stable transfection. VEGF secretion was significantly reduced in the experimental group compared with the vector and mock con-trols (P < 0.05). Values were expressed as mean ± SD.
Effect of SSTR2 on VEGF mRNA and MMP-2 mRNA expression in vitro
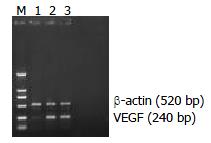
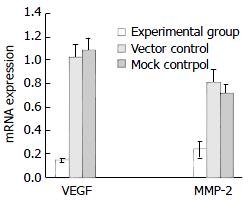
RT-PCR analysis showed that the expression of VEGF mRNA was significantly decreased in the experimental group (0.1384 ± 0.017) compared with the vector control (1.024 ± 0.11) and mock control (1.085 ± 0.105) (P < 0.05, Figures 4 and 6). Similarly, the expression of MMP-2 mRNA was also significantly reduced in the experimental group (0.2343 ± 0.07) compared with the vector control (0.806 ± 0.119) and mock control (0.714 ± 0.079) (P < 0.05, Figures 5 and 6). But there was no statistical difference either in VEGF mRNA or in MMP-2 mRNA expression between the vector control and mock control. These results suggested that the reexpression of SSTR2 gene could suppress the expression of VEGF and MMP-2 at mRNA level in vitro.
Figure 4 Weak expression of VEGF mRNA in experimental group but strong expression in vector and mock control shown by RT-PCR analysis.
The expected length of PCR products was 240 bp (VEGF) and 520 bp (β-actin). Lane M: DNA marker DL 2000, Lane 1: experimental group, Lane 2: vector control, Lane 3: mock control.
Figure 5 Weak expression of MMP-2 mRNA in experimental group but strong expression in vector andmock control shown by RT-PCR.
The expected length of PCR products was 473 bp (MMP-2) and 205 bp (β-actin). Lane M: DNA marker DL 2000, Lane 1: experimental group, Lane 2: vector control, Lane 3: mock control.
Figure 6 Significant inhibition of VEGF mRNA and MMP-2 mRNAin experimental group comparedwith vector control and mock control shown by quantification analysis of PCR products of VEGF and MMP-2 mRNA on 1.
5% agarose gel containing ethidiumbromide (P < 0.05).Valueswere expressed asmean ± SD.
Down-regulation of VEGF and MMP-2 protein expression by SSTR2
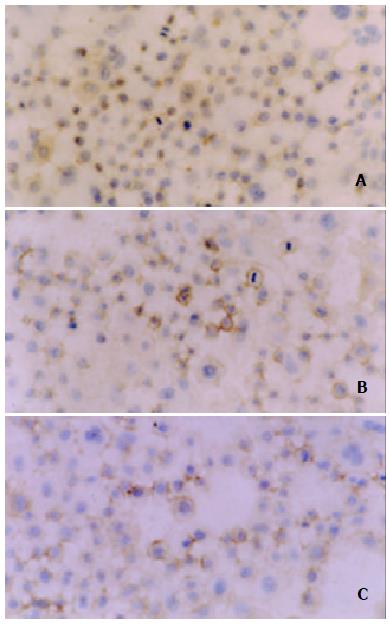
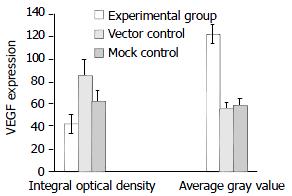
Positive staining of VEGF (Figure 7) and MMP-2 (Figure 9) was located in the cytoplasm and membrane of the cells. The immunohistochemical staining showed a significant decrease in the expression of both VEGF and MMP-2 protein in the experimental group compared with the vector control and mock control (P < 0.05). According to HPIAS-1000 and statistical analysis, the integral optical density of VEGF staining was significantly reduced in the experimental group (42.25 ± 8.6) compared with the vector control (85.75 ± 12.9) and mock control (82.6 ± 9.28) and the average gray value of VEGF staining was significantly increased in the experimental group (121.56 ± 8.43) compared with the vector control (55.72 ± 5.6) and mock control (58.48 ± 6.2) (P < 0.05, Figure 8). However, no significant difference was observed between the vector control and mock control.
Figure 7 Location of immunohistochemical staining of expression of VEGF in cytoplasm and membrane.
A: experimental group, B: vector control, C: mock control.
Figure 8 Down-regulation of VEGF protein expression by SSTR2.
The expression of VEGF was significantly decreased in experimental group compared with vector control and mock control (P < 0.05). Values were expressed as mean ± SD.
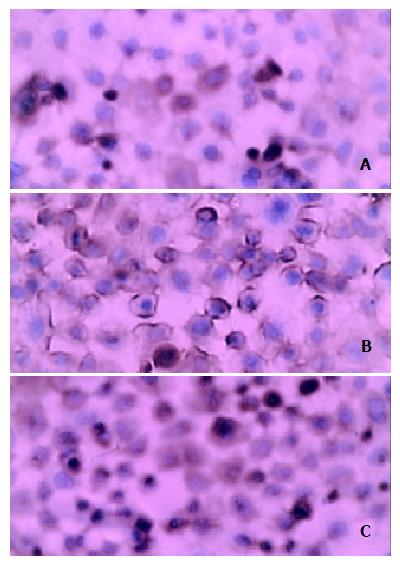
Figure 9 Location of immunohistochemical staining of expression of MMP-2 in cytoplasm and membrane.
A: experimental group, B: vector control, C: mock control (SABC × 400).
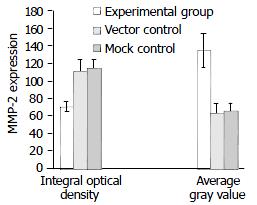
Similarly, according to HPIAS-1000 and statistical analysis, the integral optical density of MMP-2 staining was significantly reduced in the experimental group (70.5 ± 6.25) compared with the vector control (110.52 ± 13.5) and mock control (113.56 ± 9.62) and the average gray value of MMP-2 staining was significantly increased in the experimental group (134.46 ± 19.95) compared with the vector control (62.26 ± 12.68) and mock control (65.49 ± 9.16) (P < 0.05, Figure 10). However, no significant difference was observed between the vector control and mock control.
Figure 10 Down-regulation of MMP-2 protein expression by SSTR2.
The expression of MMP-2 was significantly decreased in experimental group compared with vector control and mock control (P < 0.05). Values were expressed as mean ± SD.
DISCUSSION
The properties of tumor cells to release and induce several angiogenic and antiangiogenic factors which play a crucial role in regulating EC proliferation, migration, apoptosis or survival, cell-cell and cell-matrix adhesion through different intracellular signalings, have been thought to be the essential mechanisms during tumor-induced angiogenesis[9]. The important steps during tumor angiogenesis, such as degradation of basement membrane by proteases and proliferation and migration of EC, were associated with the over-expression of angiogenic factors[9]. Since fundamental requirements of tumor growth are dependent on the blood supply, the antiangiogenic therapy of cancer represents a highly effective strategy for destroying tumors. In addition, antiangiogenic agents, if administered before a tumor develops or becomes vascular supply-dependent, would therefore theoretically act similarly to a vaccine in preventing tumor development, not just tumor growth. Similarly, transfer of antioncogene or molecules with antioncogenic properties constitutes one of the new therapeutic approaches to cancer. SSTR2 could act as an antioncogene in human pancreatic cancer cells and its antiproliferative and antimetastatic effects could occur as a consequence of an SSTR2-dependent negative feedback autocrine loop[4,27]. Furthermore, the loss of SSTR2 during pancreatic tumorigenesis[3-5] was found to be responsible for the poor therapeutic efficacy[5-7] and the aggressive behaviours of pancreatic carcinoma. However, to our knowledge, whether the abberent expression of SSTR2 is associated with pancreatic tumor angiogenesis, has not been reported as yet. But several previous investigations have shown that SS/analogues as well as SSTRs exhibited their antitumor effects through different pathways such as inhibition of release of growth factors and trophic hormones, e.g., growth hormone, insulin-like growth factor-1, insulin, gastrin, etc[2]. In CAM model, the study showed that unlabeled SS analogues inhibited angiogenesis which was proportional to the ability of the analogues to inhibit growth hormone production[8]. Moreover, Mentlein et al[30] reported that VEGF produced by cultured glioma cell lines constantly over-expressing SSTRs, especially SSTR2, was reduced to 25% to 80% by co-incubation with SS or SSTR2-selective agonists (octreotide and L-054522) in a dose-dependent manner. Interestingly, transfer of SSTR2 gene was found to restore the responsiveness of SSTR2-negative cells to SS analogues, and inhibited the tumorigenicity of pancreatic tumor cells in vitro without administration of exogenous SSTR2 ligands[4]. Similar to these, in our study, we suceesfully reexpressed exogenous human SSTR2 gene in pancreatic cancer cell line PC-3 by lipofectamine-mediated stable transfection and investigated its antiangiogenic mechanisms in the absence of SSTR2 ligand, SS.
The most commonly found angiogenic growth factors such as VEGF could contribute to the progression of many solid tumors by promoting the “angiogenic switch”[31]. In patients with pancreatic cancer, hypervascularity which was correlated with the over-expression of several angiogenic factors such as VEGF, PD-ECGF, MMP-2, etc, was significantly associated with tumor extension, lymph node status and shorter median survival time[9,14,32]. And also since VEGF via binding to its high affinity receptors (VEGFR) on EC, exerted its mitogenic effect by promoting EC proliferation and migration, resulting in tumor neovascularization[9,10], the VEGF-VEGFR system has been considered as a promising target for the development of antiangiogenic tumor therapy[33-37]. In our study, we observed a significant decrease in VEGF production levels in the cultured supernatants of the experimental group (cells reexpressed SSTR2) when compared with the vector control and mock control (P < 0.05). We further observed that the antisecretory effect of SSTR2 was similar to or even more durable than that of SS/analogues which was time- and dose-dependent[29], because in our experiment, we observed a significant inhibition of VEGF production in the cultured supernatants of the experimental group, which persisted for at least two months after stable transfection and the inhibitory rate of SSTR2 was slightly less after two months than in the first week after stable transfection, suggesting that there was a long-term induction of SS (ligand of SSTR2) by the SSTR2-dependent negative feedback autocrine loop. By contrast, Hipkin et al[38] reported that the inhibitory effects of SSTR2 gene were not permanent and stable because of its down-regulation or desensitization by long-term exposure to SS. In addition, we observed a significant inhibitory effect of SSTR2 on VEGF expression both at protein and mRNA levels in vitro detected by immunohistochemical and RT-PCR assay, respectively. All of these antisecretory events evoked by SSTR2 expression in PC-3 cell may explain the antiangiogenic effect observed in vitro.
On the other hand, up-regulation of MMPs activity favoured proteolytic degradation of the basement membrane and ECM, thereby releasing angiogenic mitogens stored within the matrix, and has been linked to tumor growth and metastasis, as well as tumor-associated angiogenesis[9]. Furthermore, the aggressive phenotype of pancreatic carcinoma has been reported to be associated with over-expression of MMP-2[21-24]. Due to the high level expression of MMP-2 in clinical and experimental models of pancreatic cancer, inhibition of MMP-2 has shown a great promise with synthetic inhibitors as antitumor agents (antiangiogenesis, antiproliferative and antimetastasis) in preclinical models[39]. Here, we observed that MMP-2 was significantly down-regulated both at protein and mRNA levels in vitro in the experimental group when compared with the vector control and mock control, suggesting that the reexpression of SSTR2 in pancreatic cancer cell line PC-3 could decrease the aggressive phenotype of pancreatic carcinoma. Similarly, Wang et al[29] reported that administration of SS analogue, octreotide, was found to inhibit the migration and invasion of gastric cancer cells in vitro and the metastasis of cancer in vivovia down-regulation of MMP-2 and VEGF expressions, thereby decreasing tumor angiogenesis.
In conlusion, our present study shows that the reexpression of SSTR2 gene can inhibit angiogenesis in pancreatic carcinoma by decreasing endogenous levels of VEGF and MMP-2, suggesting that restoration of SSTR2 in pancreatic cancer cells may offer an avenue for antiangiogenic therapy.