Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.361
Revised: May 27, 2003
Accepted: June 2, 2003
Published online: February 1, 2004
AIM: To purify the heat shock protein (HSP) 70-associated tumor peptides and to observe its non-MHC-I molecule restrictive antitumor effect.
METHODS: By ConA-sepharose affinity chromatography, ADP-agarose affinity chromatography, and DEAE anion exchange chromatography, we were able to purify HSP70-associated peptides from mouse hepatoma (HCaF) cells treated in heat shock at 42 °C. Specific active immunization and adoptive cellular immunization assay were adopted to observe the immunoprotective effect elicited by HSP70-associated peptide complexes isolated from HcaF.
RESULTS: The finally purified HSP-associated peptides had a very high purity and specificity found by SDS-PAGE and Western blot. Mice immunized with HSP70-associated peptide complexes purified from HCaF cells were protected from HCaF living cell challenge. This effect was dose dependent. Adoptive immunization of immune spleen cells of mice immunized with HSP70-associated peptide complexes could elicit immunity against HCaF challenge, and the tumor-free mice could resist repeated challenges. This effect could be continuously enhanced by repeated challenge with HCaF living cells. The tumor-free mice could tolerate the challenge for as high as 1 × 107 HCaF cells. The mice immunized once with spleen cells pulsed with HSP70-associated peptide complexes in vitro could also result in a certain adoptive immunity against HCaF.
CONCLUSION: High purity and specificity of HSP70-associated peptides could be achieved from tumor cells by the low-pressure affinity chromatography method used in this study. HSP70-associated peptide complexes derived from the HCaF can elicit non-MHC-I molecule restrictive immunoprotective effect against HCaF. This effect can be transferred by adoptive immunization to mice and enhanced by repeated challenge with HCaF live cells.
- Citation: Chen DX, Su YR, Shao GZ, Qian ZC. Purification of heat shock protein 70-associated tumor peptides and its antitumor immunity on hepatoma in mice. World J Gastroenterol 2004; 10(3): 361-365
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.361
Heat shock proteins (HSPs) are molecular chaperones which are emerging as biochemical regulators of cell growth, apoptosis, protein homeostasis and cellular targets of peptides. Numerous studies have demonstrated that HSP70 preparations derived from a tumor can elicit cancer-specific immunity against the same tumor by virtue of their ability to bind tumor-specific peptides[1-7]. Further studies indicated that tumor immunity elicited by immunization with HSP peptide complexes, including HSP70 and gp96 family, is mediated by CD8+ T lymphocytes, and its mechanism involves MHC-I class molecule restricted response which is required to channel the peptides into class I presentation pathway[8-10]. In this study, we isolated successfully HSP70-associated peptides from mouse hepatoma HCaF by low-pressure chromatography system and investigated the non-MHC-I class molecule restrictive anti-tumor immunity elicited by purified HSP70 associated peptide complexes.
Animals and tumor strain BALB/c mice (H-2d), weighing 18-20 g, were purchased from Xipuerbikai Experimental Animal Ltd, Shanghai, China. Mouse hepatoma HCaF(non-MHC-I class molecule expression) was obtained from Cancer Institute, Dalian Medical University, China.
Reagents ConA-sepharose was purchased from Pharmacia Inc. ADP, ADP-agarose from Sigma Corp. Macro-Pre DEAE support, Macro-Prep High Q from Bio-Rad Corp. Low-molecule weight standard protein and IgG of goat anti-mouse labeled with horseradish peroxidase from B.M Corp, RPMI1640 and new born bovine serum from GIBCO Corp, and anti-HSP70 McAb(mouse anti-mouse) from Wuhan Boster Corp. All other reagents used were of analytic grade.
Purification of HSP70-associated peptides The ascites of mice which had been inoculated intraperitoneally with HCaF cells for 6-7 days were used. HCaF cells were washed three times in PBS, and then suspended in RPMI1640 complete medium with water immersion at 42 °C for 12 hours. The HCaF cell pellets harvested were homogenized in hypotonic buffer (10 mM NaHCO3, 0.5 mM PMSF, pH7.1) and centrifuged at 100000 × g for 90 min at 4 °C, and the supernatant was collected. The supernatant concentrated by PEG(MW600) was applied to a ConA-sepharose column in the presence of ConA-sepharose bound buffer C (20 mM Tris-acetate, pH7.5, 0.5 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 15 mM 2 ME, 0.5 mM PMSF), and fluid was collected at a flow rate of 12 ml/h, that was the ConA-sepharose unbound protein. The fraction was dialyzed against buffer D (20 mM Tris-acetate, pH7.5, 20 mM NaCl, 3 mM MgCl2, 15 mM 2ME, 0.5 mM PMSF) overnight at 4 °C. The sample was applied to an ADP-agarose column equilibrated previously with buffer D at a flow rate of 12 ml/h. The proteins were eluted by buffer D containing 0.5M NaCl and buffer D until the protein was not detected by Bradford method. The column was eluted by 25 ml buffer D containing 3 mM ADP. The harvested elute was concentrated and dialyzed against DEAE ion-exchange buffer A (20 mM Na3PO4, 20 mM NaCl, pH7.2). The sample was applied on a DEAE column equilibrated with buffer A at a flow rate of 10 ml/h. After buffer A equilibrium for 30 min, the target protein was eluted at a linear gradient of 20 mM - 1000 mM NaCl in buffer A (20 mM Na3PO4, 1M NaCl, pH7.0, ranging from 0% - 100%). Various fractions harvested were detected with SDS-PAGE and silver staining. The fractions of HSP70 protein were collected, pooled and dried with freeze-drying, and stored at -20 °C until further use.
Identification of HSP70-associated tumor peptides HSP70 proteins were resolved on 10% SDS-PAG, subjected to electrophoresis, detected by silver staining, and blotted using mAb specific for HSP70. Manipulation of SDS-PAGE and Western blot were performed according to the method described by Sambrook et al[11]. The protein content was determined by Bradford standard curve method[12].
Active immunization assays BALB/c mice were immunized subcutaneouly with HSP70-associated peptide complexes, supernatant from homogenate of HCaF cells treated with heat shock (S-HCaF), supernatant from homogenate of liver cells treated with heat shock (S-HC), and PBS twice at weekly intervals separately and challenged by subcutaneous injection of the indicated number of HCaF living cells (5 × 104 cells in 100 ml PBS) one week after the last immunization.
Adoptive immunoprotection experiment BALB/c mice were immunized with tail vein injection of immune spleen cells (ISC, 1 × 107 cells in 200 ml PBS) of mice immunized with HSP70-associated peptides and free of tumor, twice at 5 days intervals, and challenged subcutaneouly by 5 × 104 HCaF living cells in 100 ml PBS 3 days after the last immunization. The mice with complete protection were challenged by 1 × 105 HCaF living cells again 50 days after the first challenge. The mice which tolerated the second HCaF challenge, were challenged by 1 × 107 HCaF living cells again. In another experiment, the mice were immunized by tail vein injection of spleen cells (1 × 107 cells in 200 ml PBS) pulsed with HSP70-associated peptides in vitro and challenged subcutaneouly by 5 × 104 HCaF living cells in 100 ml PBS 3 days after immunization. Corresponding control groups were set in above experiment.
Values were expressed as mean ± SD or percent (%). The data were analyzed with SPSS 8.0 software package. The results were considered statistically significant when P < 0.05.
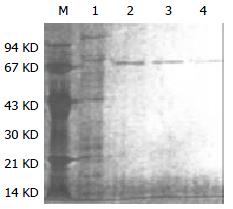
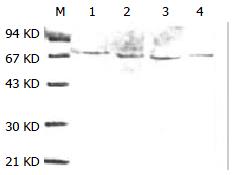
Purified HSP70-associated peptides showed one was bond on SDS-PAGE (Figure 1). Western blotting showed that molecular weight of HSP70-associated peptides purified from HCaF was about 70KD, which was consistent with the expected maker (Figure 2). The results indicated that HSP70-associated peptide complexes isolated from HCaF had a very high purity and specificity.
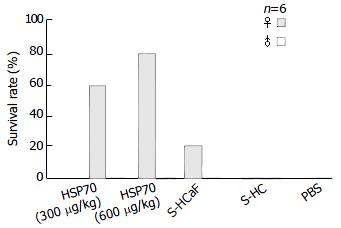
As shown in Figure 3 and Table 1, different degrees of immunoprotection against HCaF challenge could be elicited by immunization with HCaF-70 associated peptide complexes derived from HCaF. The effect on female groups was much better than that on male groups. This effect was dose-dependent. In the female groups, mice immunized with 600 μg/kg HSP70-associated peptides showed better protection than those immunized with 300 μg/kg HSP70-associated peptides. The survival rate of the two groups was 83.3% and 60%, respectively. The female mice immunized with S-HCaF also could result in a certain extent of protective effect against HCaF challenge, and the survival rate and mean survival time of tumor-bearing mice had significant differences from S-HC and PBS control groups (P < 0.01).
| Groups | Sex | No. of tumor-bearing/No. of mice challenged | Survival days of tumor-bearing (mean ± SD) | Extended survival rate (%) | |
| HSP70 | 300 μg/kg | Female | 2/6 | 48.0 ± 8.5ad | 67.4bd |
| Male | 6/6 | 31.0 ± 13.2 | 49.7c | ||
| 600 μg/kg | Female | 1/6 | 33 | ||
| Male | 6/6 | 30.7 ± 12.7 | 48.4c | ||
| S-HCaF | Female | 5/6 | 59.2 ± 10.0bd | 106.5bd | |
| Male | 6/6 | 20.3 ± 0.9 | -2.0 | ||
| S-HC | Female | 6/6 | 24.0 ± 5.8 | -16.3 | |
| Male | 6/6 | 18.5 ± 0.6 | -10.5 | ||
| PBS | Female | 6/6 | 28.7 ± 11.6 | ||
| Male | 6/6 | 20.7 ± 6.1 | |||
On the 10th day and 15th day after HCaF challenge, the tumor volume of tumor-bearing mice in both HSP70-associated peptides and S-HCaF groups was apparently smaller than that in S-HC and PBS groups (Table 2). In females, tumor weight and spleen weight of dead mice in both HSP70-associated peptides and S-HCaF groups were significantly larger than those in S-HC and PBS groups (Table 3).
| Groups | Sex | No. of tumor- | 10 days after HCaf challenge | 15 days after HCaF challenge | |||
| Size of tumor (mm3) | Inhibition rate (%) | Size of tumor (mm3) | Inhibition rate (%) | ||||
| bearing mice | |||||||
| HSP70 | 300 μg/kg | Female | 2 | 0.88bc | 97.0ac | 6.66bd | 98.5ad |
| Male | 6 | 61.98bd | 80.2bd | 384.77bc | 83.2bc | ||
| 600 μg/kg | Female | 1 | 0.45 | 98.4 | 5.74 | ||
| Male | 6 | 64.49b | 79.4bd | 515.10bc | 77.6bc | ||
| S-HCaF | Female | 5 | 3.09bc | 89.2bc | 10.21bc | 97.7bc | |
| Male | 6 | 24.27bd | 92.2bd | 501.55b | 78.2bd | ||
| S-HC | Female | 6 | 30.00 | -4.5 | 402.17 | 8.2 | |
| Male | 6 | 204.89 | 34.5 | 1554.30 | 32.3 | ||
| PBS | Female | 6 | 28.70 | 438.17 | |||
| Male | 6 | 312.83 | 2295.52 | ||||
| Groups | Female | Male | |||||
| No. of tumor-bearing mice | Tumor | Spleen | No. of tumor-bearing mice | Tumor | Spleen | ||
| wt. (g) | wt. (g) | wt. (g) | wt. (g) | ||||
| HSP70 | 300 μg/kg | 2 | 14.40 ± 6.22bce | 0.93 ± 0.18bde | 6 | 7.06 ± 2.59 | 0.19 ± 0.04 |
| 600 μg/kg | 1 | 6.5 | 0.4 | 6 | 7.51 ± 2.33 | 0.20 ± 0.11 | |
| S-HCaF | 6 | 8.68 ± 1.65a | 0.53 ± 0.22be | 6 | 5.06 ± 0.71 | 0.24 ± 0.10 | |
| S-HC | 5 | 4.72 ± 1.65 | 0.17 ± 0.08 | 6 | 4.98 ± 1.04 | 0.14 ± 0.02 | |
| PBS | 6 | 6.78 ± 1.34 | 0.25 ± 0.06 | 6 | 6.30 ± 2.18 | 0.12 ± 0.07 | |
Adoptively transferred immune spleen cells of mice, which had been immunized with HSP70-associated peptides and were free of tumor, could provoke immunoprotection against HCaF challenge. The survival rate of ISC-immunized mice was 75%, and the mean survival time of tumor-bearing mice was significantly prolonged compared with both non-immune spleen cells (SC) group and challenge control (Table 4).
| Group | Survival rate (%) | Survival days of | Extended | Tumor wt. (g) | Spleen wt.(g) | |
| (No. of death/No. of mice challenged) | tumor-bearing mice (mean ± SD) | survival rate (%) | (mean ± SD) | (mean ± SD) | ||
| ISC | 75 | (2/8)a | 50.5 ± 9.2b | 71.0b | 14.92 ± 0.42bc | 0.39 ± 0.01bc |
| SC | 0 | (8/8) | 36.8 ± 12.9 | 24.6 | 7.73 ± 2.14 | 0.18 ± 0.05 |
| Chal. control | 0 | (8/8) | 29.5 ± 3.9 | 0 | 7.35 ± 1.16 | 0.21 ± 0.05 |
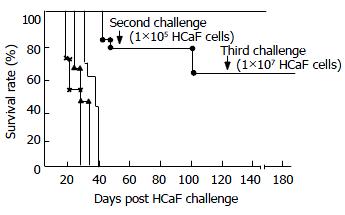
The mice which tolerated HCaF challenge were challenged by 1 × 105 HCaF living cells, and the survival rate was 83.3%, while all of the challenge controls died within 27.5 days. The mice which tolerated the second challenge could tolerate repeated challenges for as high as 1 × 107 HCaF living cells, while all the challenge controls died within 22 days (Figure 4).
The mice immunized once with spleen cells pulsed with HSP70-associated peptides in vitro could lead to a certain extent of protection against HCaF challenge. Although the survival rate of mice was only 20%, the mean survival time of tumor-bearing mice was 42 days, all the mice treated with S-HCaF, S-HC, SC or PBS died within 33 days.
Intracellular HSP70 is very low in content of cells and makes up approximately 0.01% of the cell wet weight. At present, ConA-sepharose affinity chromatography or ADP-agarose affinity chromatography in combination with fast protein liquid chromatography[13,14] has been the typical method for purifying HSP70. The recovery of HSP70 isolated by the above methods was lower than 50 mg/L cell pellet[4]. The purification protocol used in our experiment resulted in a relatively high recovery compared with the traditional method of HSP70 purification, being for 50-100 mg/L wet weight of cell pellet. This purification method might be used as a universal technique due to its easy and reproducible isolation of antigenic HSP from other tissues of different sources.
Numerous investigations have shown that HSP itself had no antigenicity and its immunogenicity has been attributed to the peptide chaperoned carried by itself[1-4]. In this experiment, tumor rejection assay demonstrated that HSP70 purified from HCaF could elicit tumor immunity. We therefore conclude that purified HSP70 identified by both SDS-PAGE and Western blot should be regarded as HSP70-associated tumor peptides. Our experiment indicated that HSP70-associated peptides derived from HCaF could elicit anti-tumor immunity. Mice immunized with 600 μg/kg HSP70-associated peptides showed better protection than those immunized with 300 μg/kg. This effect was dose-dependent, and was consistent with other reports[4]. In this study, we found the transferred immune spleen cells of mice immunized with HSP70-associated peptide complexes could elicit immunity against HCaF challenge, and the tumor-free mice could resist repeated HCaF challenges. This effect could be continuously enhanced by repeated challenges with HCaF living cells. The mice so treated could tolerate a challenge for as high as 1 × 107 HCaF cells. Our results demonstrated that adoptively transferred immune spleen cells immunized with HSP70-associated tumor peptides could result in immunoprotection against the same tumor. This evidence indicates that anti-tumor immunity elicited by HSP70-associated peptides has a considerable stability of immunoprotection and specific immunologic memory.
It has been generally believed that tumor immunity elicited by immunization with exogenous HSP70-peptide complexes is mediated by antigen-presenting cells and presented by MHC class I molecules[15-24]. It is worth pointing out that the tumor model of HCaF used in our experiment did not express MHC class I molecule protein. Therefore, the tumor immunity elicited by HSP70-associated peptides derived from HCaF might not be mediated by CD8+ CTL. Several studies have shown that HSP70-associated peptides could directly activate γδ+T lymphocytes or nature killer cells as superantigen without being dependent on the stimulation of MHC-Ia and I b class molecules[25-30]. It is possible that HSP70-associated peptides derived from HCaF can elicit antitumor immunity in a similar manner.
In addition, we found that the supernatants of HCaF cell homogenate could also result in a certain tumor immunity. This effect might be related to the expression of HSP in HCaF cells induced by heat shock.
Compared spleen weight of tumor-bearing mice in various groups, the mean spleen weight of tumor-bearing mice in HSP70-associated peptide complexes group was significantly higher than that in the controls. Our results further showed that adoptively transferred spleen cells pulsed with purified HSP70-associated peptides could also provoke a certain protection against HCaF challenge. These results indicated that spleen cells might play an important role in tumor immunity mediated by HSP70-associated peptides.
It is of great interest to note that the protective effect in the female mice immunized with HSP70-associated peptide complexes was significantly better than that in the male group. The difference may be associated with estrogens, its mechanisms remain to be explored further.
Edited by Ma JY and Wang XL
| 1. | Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407-3411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 322] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Palladino MA, Srivastava PK, Oettgen HF, DeLeo AB. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. Cancer Res. 1987;47:5074-5079. [PubMed] |
| 3. | Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 452] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Ciupitu AM, Petersson M, Kono K, Charo J, Kiessling R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell responses. Cancer Immunol Immunother. 2002;51:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Basu S, Srivastava PK. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones. 2000;5:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Przepiorka D, Srivastava PK. Heat shock protein--peptide complexes as immunotherapy for human cancer. Mol Med Today. 1998;4:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Meng SD, Gao F, Tien P. [Role of heat shock protein-peptide complexes on tumor and infectious diseases immunity]. Shengwu Gongcheng Xuebao. 2000;16:425-428. [PubMed] |
| 9. | Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D. Cross-presentation of glycoprotein 96-associated antigen on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965-1974. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A labo-ratory munual. Second Edition. USA: Cold Spring Harbor Labora-tory Press 1989; 18.47-18.75. |
| 12. | Marshak DR, Kadonaga JT, Burgess RR, Knuth MW, Brennan JR, Lin SH. Strategies for protein purification and characterization: A laboratory course manual. Beijing: China Science Press 1999; 158-159. |
| 13. | Peng P, Ménoret A, Srivastava PK. Purification of immunogenic heat shock protein 70-peptide complexes by ADP-affinity chromatography. J Immunol Methods. 1997;204:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 615] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 387] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398-5403. [PubMed] |
| 17. | Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 796] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 18. | Dressel R, Lübbers M, Walter L, Herr W, Günther E. Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J Immunol. 1999;29:3925-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, Hizuta A, Tanaka N, Srivastava PK, Nakayama E. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303-1309. [PubMed] |
| 21. | Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146-13151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 188] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 407] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Heike M, Noll B, Meyer zum Büschenfelde KH. Heat shock protein-peptide complexes for use in vaccines. J Leukoc Biol. 1996;60:153-158. [PubMed] |
| 24. | Ojcius DM, Delarbre C, Kourilsky P, Gachelin G. Major histocompatibility complex class I molecules and resistance against intracellular pathogens. Crit Rev Immunol. 1994;14:193-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kaur I, Voss SD, Gupta RS, Schell K, Fisch P, Sondel PM. Human peripheral gamma delta T cells recognize hsp60 molecules on Daudi Burkitt's lymphoma cells. J Immunol. 1993;150:2046-2055. [PubMed] |
| 26. | Thomas ML, Samant UC, Deshpande RK, Chiplunkar SV. gammadelta T cells lyse autologous and allogenic oesophageal tumours: involvement of heat-shock proteins in the tumour cell lysis. Cancer Immunol Immunother. 2000;48:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Multhoff G, Botzler C, Issels R. The role of heat shock proteins in the stimulation of an immune response. Biol Chem. 1998;379:295-300. [PubMed] |
| 28. | Botzler C, Li G, Issels RD, Multhoff G. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones. 1998;3:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia. 2002;18:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |