Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.337
Revised: November 12, 2003
Accepted: November 19, 2003
Published online: February 1, 2004
AIM: To investigate the ability of protease inhibitors to modulate histamine release from human colon mast cells.
METHODS: Enzymatically dispersed cells from human colon were challenged with anti-IgE or calcium ionophore A23187 in the absence or presence of tryptase and chymase inhibitors, and histamine release was determined.
RESULTS: IgE dependent histamine release from colon mast cells was inhibited by up to approximately 37%, 26% and 36.8% by chymase inhibitors Z-Ile-Glu-Pro-Phe-CO2Me (ZIGPFM), N-Tosyl-L-phenylalanyl-chloromethyl ketone (TPCK), and α1-antitrypsin, respectively. Similarly, inhibitors of tryptase leupeptin, N-tosyl-L-lysine chloromethyl ketone (TLCK), lactoferrin and protamine were also able to inhibit anti-IgE induced histamine release by a maximum of some 48%, 37%, 40% and 34%, respectively. Preincubation of these inhibitors with cells for 20 min before challenged with anti-IgE had small effect on the inhibitory actions of these inhibitors on colon mast cells. A specific inhibitor of aminopeptidase amastatin had no effect on anti-IgE induced histamine release. The significant inhibition of calcium ionophore induced histamine release was also observed with the inhibitors of tryptase and chymase examined. Apart from leupeptin and protamine, the inhibitors tested by themselves did not stimulate colon mast cells.
CONCLUSION: It was demonstrated that both tryptase and chymase inhibitors could inhibit IgE dependent and calcium ionophore induced histamine release from dispersed colon mast cells in a concentration dependent of manner, which suggest that they are likely to be developed as a novel class of anti-inflammatory drugs to treat chronic of colitis in man.
- Citation: He SH, Xie H. Modulation of histamine release from human colon mast cells by protease inhibitors. World J Gastroenterol 2004; 10(3): 337-341
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/337.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.337
It has been reported that mast cells and their inflammatory mediators are closely associated with a number of intestinal diseases including idiopathic inflammatory bowel disease[1], chronic ulcerative colitis[2], Crohn’s disease[3] and collagenous colitis[4]. Through release their proinflammatory mediators including histamine, tryptase, chymase, heparin and some cytokines[5], mast cells actively participate in the pathogenesis of these intestinal diseases.
As a proinflammatory mediator, histamine is selectively located in the granules of human mast cells and basophils and released from these cells upon degranulation. To date, a total of four histamine receptors H1, H2, H3 and H4 have been discovered[6] and the first three of them are located in human gut[7,8], which prove that there are some specific targets that histamine can work on in intestinal tract. Indeed, increased levels of histamine or enhanced histamine metabolism have been observed in collagenous colitis, food allergy[9], Crohn’s disease[10], ulcerative colitis[10,11] and allergic enteropathy[11], indicating that this mediator is involved in the pathogenesis of these diseases.
For more than four decades, histamine has been widely used as a marker of mast cell degranulation in vitro, and numerous anti-allergic drugs such as sodium cromoglycate, lodoxamide, salbutamal, ketotifen, terfenadine and cetirizine[12,13] and salmeteral[14] were reported to be able to inhibit anti-IgE induced histamine release from human mast cells. In recent years, inhibitors of tryptase[15,16] and chymase[17] have been discovered to possess the ability to inhibit histamine release from human skin, tonsil and synovial mast cells[18], suggesting these inhibitors are likely to be developed as a novel class of mast cell stabilizers. However, little is known of the actions of tryptase and chymase inhibitors on histamine release from human colon mast cells. We therefore investigated the effects of these two groups of inhibitors on IgE dependent or independent histamine release from human colon mast cells in the current study.
Human colon tissue was obtained from patients with carcinoma of colon at colectomy. Only macroscopically normal tissue was used for the study. After removal of fat, tissue was washed and chopped finely with scissors into fragments of 0.5-2.0 mm3, and then incubated with 1.5 mg/mL collagenase (Sigma) and 0.75 mg/mL hyaluronidase (Sigma) in minimum essential medium (MEM) containing 2% fetal calf serum (1 g colon/10 mL buffer) for 70 min at 37 °C. Dispersed cells were separated from undigested tissue by filtration through nylon gauze (pore size 100 µm diameter), washed and maintained in MEM (Gibco) (containing 10% FCS, 200 U/mL penicillin, 200 µg/mL streptomycin) on a roller overnight at room temperature. Mast cell purity, as determined by light microscopy after stained by alcine blue, ranged from 3.5% to 5.4%.
The challenge procedure was performed as described previously[19]. Dispersed cells were resuspended in HEPES buffered salt solution (HBSS, pH7.4) with CaCl2 and MgCl2 (complete HBSS), and 100 µL aliquots containing 4-6 × 103 mast cells were added to a 50 µL anti-IgE (Serotec, UK), calcium ionophore (Sigma), or inhibitor in complete HBSS and incubated for 15 min at 37 °C. The reaction was terminated by addition of 150 µL ice cold incomplete HBSS and the tubes were centrifuged immediately (500 g, 10 min, 4 °C). All experiments were performed in duplicate. For the measurement of total histamine concentration, in certain tubes the suspension was boiled for 6 min. Supernatants were stored at -20 °C until histamine concentrations were determined.
For some experiments, protease inhibitor was preincubated with cells for 20 min before anti-IgE or calcium ionophore being added. Protease inhibitor and anti-IgE or calcium ionophore were also added to cells at the same time (no preincubation period). Data were expressed as the percentage of inhibition of histamine release, taking into account of histamine release in the presence and absence of the inhibitor. As for our previous experiments, the optimal histamine release from colon mast cells was induced by 10 µg/mL anti-IgE or 1 µg/mL calcium ionophore[20], and therefore they were chosen as standard concentrations throughout the study.
Histamine concentrations were determined using a glass fibre-based fluorometric assay[15]. The procedure involved the binding of histamine to a glass-fiber matrix (RafLab, Copenhagen, Denmark) and its detection spectrophotometrically with Perkin-Elmer LS 2 detector (Denmark) following addition of o-phthaldialdehyde (OPT). Histamine release was expressed as a percentage of total cellular histamine levels, and corrected for the spontaneous release measured in tubes in which cells had been incubated with the HBSS diluent alone.
Statistical analyses were performed with SPSS software. Data were expressed as mean ± SEM. Where analysis of variance indicated significant differences between groups with ANOVA, for the preplanned comparisons of interest, Student’s t test was applied. For all analyses, P < 0.05 was taken as statistically significant.
At 15 min following incubation, leupeptin at concentration 200 µmol/mL and protamine at 100 µg/mL were able to provoke small but nevertheless significant histamine release from colon mast cells (Table 1). The same concentration of leupeptin was also capable of eliciting histamine release following a 35 min incubation period (Table 2). All the other protease inhibitors tested had no stimulatory action on colon mast cells. Leupeptin and protamine at all other concentrations did not induce a significant histamine release from colon mast cells. In the same experiments, anti-IgE and calcium ionophore were able to induce up to 11% and 21.8% net histamine release, respectively.
| Compound | Concentration | Net histamine release (%) |
| ZIGPFM | 0.001 μmol/mL | -0.9 ± 0.8 |
| 0.01 μmol/mL | 0 ± 1.1 | |
| 0.1 μmol/mL | 0.8 ± 0.6 | |
| 1.0 μmol/mL | 0.2 ± 1.2 | |
| TPCK | 0.08 μmol/mL | 3.1 ± 2.0 |
| 0.8 μmol/mL | 2.1 ± 1.0 | |
| 8.0 μmol/mL | 2.0 ± 1.2 | |
| 80 μmol/mL | 0.7 ± 1.1 | |
| α1-antitrypsin | 1.0 μmol/mL | 0.9 ± 0.5 |
| 10 μmol/mL | 1.1 ± 0.6 | |
| 30 μmol/mL | 1.5 ± 0.6 | |
| Lactoferrin | 1.0 μmol/mL | -1.0 ± 0.8 |
| 10 μmol/mL | 0.8 ± 0.6 | |
| 30 μmol/mL | 0.5 ± 1.0 | |
| TLCK | 0.1 μmol/mL | 1.7 ± 2.1 |
| 1.0 μmol/mL | 1.1 ± 0.6 | |
| 10 μmol/mL | 2.7 ± 1.7 | |
| 100 μmol/mL | 3.5 ± 1.2 | |
| Amastatin | 0.1 μmol/mL | 1.5 ± 0.4 |
| 1.0 μmol/mL | 2.3 ± 0.6 | |
| 10 μmol/mL | 3.9 ± 0.6 | |
| Leupeptin | 2.0 μmol /mL | 1.2 ± 0.7 |
| 20 μmol /mL | 2.0 ± 1.4 | |
| 60 μmol /mL | 0.6 ± 0.5 | |
| 200 μmol /mL | 4.7 ± 0.8a | |
| Protamine | 0.1 μg/mL | 1.7 ± 1.3 |
| 1.0 μg/mL | 1.9 ± 1.4 | |
| 10 μg/mL | 0.2 ± 2.1 | |
| 100 μg/mL | 4.5 ± 0.5a | |
| Anti-IgE | 10 μg/mL | 11 ± 2.7a |
| Calcium ionophone | 1.0 μg/mL | 18 ± 3.6a |
| Compound | Concentration | Net histamine release (%) |
| ZIGPFM | 0.001 μmol/mL | 0.3 ± 0.3 |
| 0.01 μmol/mL | 1.0 ± 0.8 | |
| 0.1 μmol/mL | 1.8 ± 1.3 | |
| 1.0 μmol/mL | 1.9 ± 0.6 | |
| TPCK | 0.8 μmol/mL | 0.9 ± 2.1 |
| 8.0 μmol/mL | 1.8 ± 1.3 | |
| 80 μmol/mL | 1.7 ± 3.6 | |
| TLCK | 1.0 μmol/mL | 0 ± 2.4 |
| 10 μmol/mL | 0.7 ± 1.9 | |
| 100 μmol/mL | 0.7 ± 2.2 | |
| Amastatin | 0.1 μmol/mL | 1.0 ± 1.7 |
| 1.0 μmol/mL | -1.9 ± 3.3 | |
| 10 μmol/mL | 0 ± 3.6 | |
| Leupeptin | 2.0 μmol/mL | 1.0 ± 0.7 |
| 20 μmol/mL | 1.2 ± 0.7 | |
| 60 μmol/mL | 0 ± 0.7 | |
| 200 μmol/mL | 5.1 ± 0.5a | |
| Protamine | 1.0 μg/mL | 1.2 ± 2.9 |
| 10 μg/mL | 1.0 ± 2.5 | |
| Anti-IgE | 10 μg/mL | 10.1 ± 2.5a |
| Calcium ionophone | 1.0 μg/mL | 21.8 ± 3.8a |
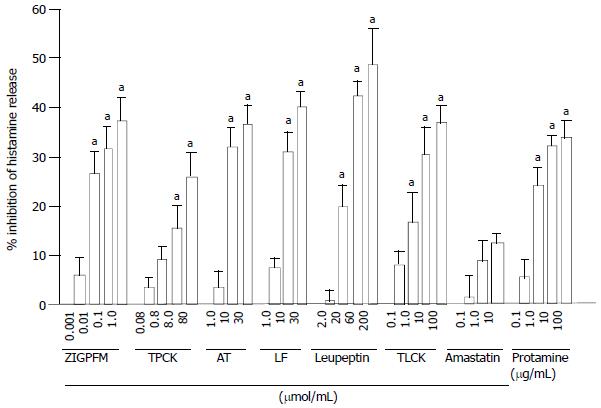
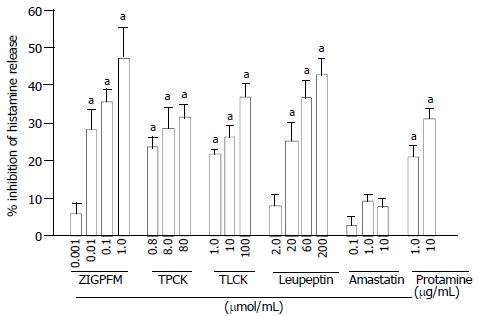
The concentration dependent inhibition of anti-IgE induced release of histamine from colon mast cells was observed when anti-IgE and various concentrations of chymase inhibitors ZIGPFM, TPCK, and α1-antitrypsin were added to cells at the same time. Up to approximately 37%, 26% and 36.8% inhibition of IgE dependent histamine release were achieved with ZIGPFM, TPCK, and α1-antitrypsin, respectively (Figure 1). Preincubation of ZIGPFM and TPCK with cells for 20 min before challenged with anti-IgE was able to slightly enhance their inhibitory actions (Figure 2).
The inhibitors of tryptase leupeptin, TLCK, lactoferrin and protamine were also able to inhibit anti-IgE induced histamine release in a concentration dependent manner, and a maximum of 48%, 37%, 40% and 34% inhibition was achieved with 200 µmol/mL leupeptin, 100 µmol/mL TLCK, 30 µmol/mL lactoferrin and 100 µg/mL protamine, respectively (Figure 1). In contrast to inhibitors of chymase, preincubation of inhibitors of tryptase with cells for 20 min before the addition of anti-IgE had little effect on their abilities to inhibit anti-IgE induced histamine release (Figure 2). A specific inhibitor of aminopeptidase, amastatin had no effect on anti-IgE induced histamine release (data not shown).
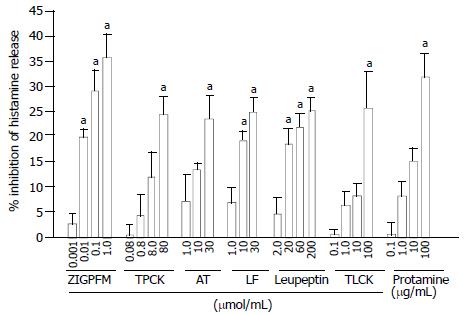
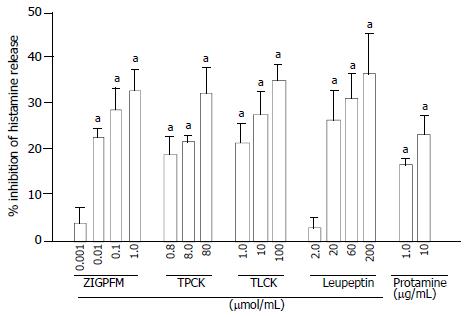
The concentration dependent inhibition of calcium ionophore induced histamine release from colon mast cells was observed when calcium ionophore and various concentrations of chymase inhibitors ZIGPFM, TPCK, and α1-antitrypsin were added to cells at the same time. Up to approximately 35%, 24% and 23.6% inhibition of IgE dependent histamine release were achieved with ZIGPFM, TPCK, and α1-antitrypsin, respectively (Figure 3). Preincubation of TPCK with cells for 20 min before challenged with calcium ionophore slightly enhanced its inhibitory ability, whereas the same treatment did not improve the inhibitory ability of ZIGPFM (Figure 4).
Calcium ionophore stimulated histamine release was also reduced by addition of the various concentrations of inhibitors of tryptase to cells. Leupeptin, TLCK, lactoferrin and protamine were able to inhibit calcium ionophore stimulated histamine release by up to approximately 25%, 26%, 25% and 32%, respectively when they were added to cells together with calcium ionophore (Figure 3). The extent of inhibition by leupeptin and TLCK was increased when colon mast cells were preincubated with them for 20 min before calcium ionophore was added. However, the same treatment failed to improve the inhibitory action of protamine (Figure 4).
We have found that inhibitors of tryptase and chymase were able to inhibit anti-IgE and calcium ionophore induced histamine release from dispersed human colon mast cells, which may indicate a potential of a novel therapy for inflammatory bowel disease or other mast cell related intestinal diseases.
Up to approximately 37% inhibition of IgE dependent histamine release from colon mast cells was observed with inhibitors of chymase, indicating that a chymase activity was involved in the process of IgE dependent gut mast cell degranulation. This was consistent with our previous findings with human skin, lung[17] and synovium tissues[18], which demonstrated that chymase was involved in the mast cell activation-degranulation process. Comparing mast cells from different human tissues, the order of extent of maximum inhibition by chymase inhibitors was skin (82%) > lung (80%) > synovium (69%) > colon (37%). This might represent a novel type of mast cell heterogeneity, and could also be resulted from an inhibitor of chymase, chymostatin was not used for colon cells, but for the cells from other tissues.
Similar to chymase inhibitors, tryptase inhibitors inhibited up to some 48% anti-IgE induced histamine release from colon mast cells, which implicated that a tryptase activity was likely to be involved in the process of colon mast cell degranulation. Once again, this was consistent with our previous findings with cells from other human tissues including skin, tonsil[15] and synovium[18]. Comparing mast cells from different human tissues, the order of extent of maximum inhibition by tryptase inhibitors was skin (90%) > synovium (70%) > colon (48%) > tonsil (35%).
Since the majority of these inhibitors at the concentrations used in the current study were able to inhibit more than 95% tryptase or chymase activity in enzyme assays[20], the incomplete inhibition of histamine release from mast cells may suggest that some pathways other than tryptase and chymase pathways are involved in the anti-IgE induced degranulation of gut mast cells. A specific inhibitor of aminopeptidase amastatin, which did not inhibit chymotrypsin and trypsin activities[21], was used as an irrelevant protease inhibitor control. It had no significant effects on anti-IgE induced histamine release from colon mast cells, which proved the specificity of actions of tryptase and chymase inhibitors on mast cells. The observation that preincubation of the inhibitors with cells for 20 min before challenged with anti-IgE had little impact on inhibition of IgE dependent histamine release was unexpected, nevertheless it may suggest that the actions of these inhibitors are rather rapid and the involvement of tryptase and chymase activities in anti-IgE induced histamine release is likely at the downstream site of the degranulation process. Since calcium ionophore is a calcium carrier that can help to elevate the calcium concentration in cytoplasms of mast cells, and therefore acts on the downstream site of the process of mast cell degranulation, the inhibition of calcium ionophore induced histamine release by the inhibitors of tryptase and chymase in the current study might also suggest that the involvement of tryptase and chymase activities in mast cell degranulation process was at the downstream site and most likely after influx of calcium ions into mast cells. The evidence that tryptase and chymase are sited in the granules of mast cells in their fully active form supports further the likelihood that these two mast cell serine proteases are involved in IgE dependent activation of colon mast cells.
Over the years, many compounds including sodium cromoglycate, lodoxamide, salbutamal, ketotifen, terfenadine and cetirizine have been recognized as mast cell stabilizers or histamine receptor antagonists, and used as anti-allergic drugs in clinical practice. However, only less than 40% inhibition of IgE dependent mast cell degranulation could be achieved with these compounds, much less than that with inhibitors of tryptase and chymase in the similar experimental system[15,17]. Moreover, mast cell stabilizer drug ketotifen was successfully used to treat acute ulcerative colitis[22] and Crohn’s disease[23]. These strongly suggest that inhibitors of tryptase and chymase are likely to become a novel class of anti-inflammatory drugs with their anti-inflammatory actions and mast cell stabilizing properties.
In conclusion, inhibitors of both tryptase and chymase are able to inhibit anti-IgE dependent and calcium ionophore induced histamine release from colon mast cells, indicating that they are likely to be developed as a novel class of anti-inflammatory drugs to treat chronic colitis in man.
Edited by Wang XL Proofread by Zhu LH
| 1. | Fox CC, Lichtenstein LM, Roche JK. Intestinal mast cell responses in idiopathic inflammatory bowel disease. Histamine release from human intestinal mast cells in response to gut epithelial proteins. Dig Dis Sci. 1993;38:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002;104:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Nishida Y, Murase K, Isomoto H, Furusu H, Mizuta Y, Riddell RH, Kohno S. Different distribution of mast cells and macrophages in colonic mucosa of patients with collagenous colitis and inflammatory bowel disease. Hepatogastroenterology. 2002;49:678-682. [PubMed] |
| 4. | Schwab D, Raithel M, Hahn EG. Evidence for mast cell activation in collagenous colitis. Inflamm Res. 1998;47 Suppl 1:S64-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Walls AF, He SH, Buckley MG, McEuen AR. Roles of the mast cell and basophil in asthma. Clin Exp Allergy Rev. 2001;1:68-72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Repka-Ramirez MS. New concepts of histamine receptors and actions. Curr Allergy Asthma Rep. 2003;3:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Bertaccini G, Coruzzi G. An update on histamine H3 receptors and gastrointestinal functions. Dig Dis Sci. 1995;40:2052-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Rangachari PK. Histamine: mercurial messenger in the gut. Am J Physiol. 1992;262:G1-13. [PubMed] |
| 9. | Schwab D, Hahn EG, Raithel M. Enhanced histamine metabolism: a comparative analysis of collagenous colitis and food allergy with respect to the role of diet and NSAID use. Inflamm Res. 2003;52:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Winterkamp S, Weidenhiller M, Otte P, Stolper J, Schwab D, Hahn EG, Raithel M. Urinary excretion of N-methylhistamine as a marker of disease activity in inflammatory bowel disease. Am J Gastroenterol. 2002;97:3071-3077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG. Mucosal histamine content and histamine secretion in Crohn's disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol. 1995;108:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Okayama Y, Church MK. Comparison of the modulatory effect of ketotifen, sodium cromoglycate, procaterol and salbutamol in human skin, lung and tonsil mast cells. Int Arch Allergy Immunol. 1992;97:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Okayama Y, Benyon RC, Lowman MA, Church MK. In vitro effects of H1-antihistamines on histamine and PGD2 release from mast cells of human lung, tonsil, and skin. Allergy. 1994;49:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Butchers PR, Vardey CJ, Johnson M. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br J Pharmacol. 1991;104:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289-297. [PubMed] |
| 16. | He S, McEuen AR, Blewett SA, Li P, Buckley MG, Leufkens P, Walls AF. The inhibition of mast cell activation by neutrophil lactoferrin: uptake by mast cells and interaction with tryptase, chymase and cathepsin G. Biochem Pharmacol. 2003;65:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | He S, Gaça MD, McEuen AR, Walls AF. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther. 1999;291:517-523. [PubMed] |
| 18. | He S, Gaça MD, Walls AF. The activation of synovial mast cells: modulation of histamine release by tryptase and chymase and their inhibitors. Eur J Pharmacol. 2001;412:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | He SH, Li P. Mast cell activation and histamine measurement. Huaxi Yikedaxue Xuebao. 2002;33:586-588. |
| 20. | He SH, Xie H, He YS. Induction of tryptase and histamine release from human colon mast cells by IgE dependent or independent mechanisms. World J Gastroenterol. 2004;10:319-322. [PubMed] |
| 21. | He SH, Chen P, Chen HQ. Modulation of enzymatic activity of human mast cell tryptase and chymase by protease inhibitors. Acta Pharmacol Sin. 2003;24:923-929. [PubMed] |
| 22. | Rich DH, Moon BJ, Harbeson S. Inhibition of aminopeptidases by amastatin and bestatin derivatives. Effect of inhibitor structure on slow-binding processes. J Med Chem. 1984;27:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 174] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Jones NL, Roifman CM, Griffiths AM, Sherman P. Ketotifen therapy for acute ulcerative colitis in children: a pilot study. Dig Dis Sci. 1998;43:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Marshall JK, Irvine EJ. Ketotifen treatment of active colitis in patients with 5-aminosalicylate intolerance. Can J Gastroenterol. 1998;12:273-275. [PubMed] |