Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3643
Revised: April 6, 2004
Accepted: April 13, 2004
Published online: December 15, 2004
AIM: Intestinal parasitic diseases are commonly accompanied with diarrhoeal symptoms and allergic reactions. Eosinophilia occurs as a result of IL-5 synthesized from Th2 cells during allergic reactions. IL-5 acts as a factor activating eosinophils. The aim of this study was to compare the IL-5 cytokine measurements in serum samples and cell cultures. And also to compare eosinophilia observed in helminth infections and protozoon infections accompanied with allergy.
METHODS: Twenty-three patients who presented with diarrhoeal symptoms and allergic complaints were tested positive for intestinal parasites, as well as 21 controls with allergic complaints who did not have any intestinal parasites were included in this study. IL-5 production in in vitro cell cultures prepared by using phytohemaglutinin (PHA) to stimulate peripheral blood mononuclear cells (PBMC) obtained from the blood samples taken from these patients were compared with the IL-5 level in serum. Furthermore, the IL-5 production in protozoon and helminth infections was also compared. Absolute eosinophil values in 1 mm³ of blood were calculated by means of peripheral smear in both groups within the scope of the study.
RESULTS: Parasites such as helminth detected in 15 (65.2%) and protozoon in 8 (34.8%) of the patients were included in this study. As regards the values of the sera in both patients with parasite infection and controls, the IL-5 production was found to be higher in the cell culture supernatant (P < 0.001 and P < 0.05). When the IL-5 level of the patients with helminth parasites was compared with that of those with protozoon, it was determined that the IL-5 level in serum was more significant in the patients with protozoon than in those with helminth (P < 0.05). In the study group, the patients were found to have parasites, the percentage of eosinophil was 7.0% compared to 6.5% in the control group. Thus, there was no significant difference between the eosinophil values (P > 0.05).
CONCLUSION: It was found that IL-5 cytokine levels in serum samples from the patients with helminth and protozoon displayed more measurable values as compared to the IL-5 levels after stimulation with mitogen. It is concluded that IL-5 acts as a triggering factor in the toxiallergic complaints commonly seen in helminth and protozoon infections.
- Citation: Ustun S, Turgay N, Delibas SB, Ertabaklar H. Interleukin (IL) 5 levels and eosinophilia in patients with intestinal parasitic diseases. World J Gastroenterol 2004; 10(24): 3643-3646
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3643.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3643
Gastrointestinal (GI) parasites play an important role in the allergic and hypersensitive reactions. The prevalence of intestinal protozoans and helminths in stool samples of individuals with allergic symptoms was evaluated as a possible link between parasites and allergy[1]. A wide variety of clinical findings related to the whole body are encountered in parasitic diseases. Hypersensitive reactions may start out in the host against the chemical substances excreted by the parasites. In addition, allergic reactions may occur[2].
There has been an increase in the prevalence of allergic diseases in the past few decades. T helper 2 (Th2) responses associated with allergic diseases[3]. Th2 cells elaborate cytokines such as IL-5 which works with toxic mediators of innate immune cells. Th2 cytokine involvement in allergy makes these cytokines attractive therapeutic targets, which protect against gastrointestinal worms[4,5]. Immunological interventions could be designed to avoid induction of pathology while retaining protective responses[6].
It has been shown that strong Th2 cytokine response occurs especially during the chronic phase of helminth infections. Allergic reactions triggered by this Th2 response are more commonly seen in helminth infections, and hypersensitive reactions against the chemical substances secreted by helminths may occur in some people[7-9]. Long-lived parasites such as helminths, however, are more remarkable for their ability to downregulate host immunity, protecting themselves from elimination and minimizing severe pathology in the host[10].
Allergic reactions may also be observed in protozoon diseases. Ameobiasis, giardiasis, Blastocystis were reported as the etiologic factors causing allergy in animals and human models in some publications[1-11].
Eosinophilia is caused by the effect of IL-5 synthesized from the Th2 cells. IL-5 is the most important cytokine in the transformation and development of eosinophils, and acts as an “eosinophil activator”. One of the significant causes of the increase in the amount of eosinophils in blood is parasitic diseases. Toxiallergic effects of certain parasites on the host’s organism lead to an increase especially in eosinophil numbers. Eosinophils are effectors against parasitic targets. Both sets of diseases are associated with a polarized Th2-type immune response, typified by reactive cell types (eosinophils and mast cells)[12,13].
It is suggested therefore that the function of IL-5 and eosinophils is to protect against repeated exposure to gastrointestinal parasites. On the other hand the eosinophilia observed may represent an immunopathological rather than a protective response and may merely be a consequence of the generalized inflammation induced by the Th2 response following infection with parasites. Th2 response is essential for the expulsion of GI helminths[14].
Eosinophils are also responsible for considerable pathology in mammals because they are inevitably present in large numbers in inflammatory lesions associated with helminth infections or allergic conditions[15,16].
On the other hand current evidence shows a strong association between colonic infection and inflammation with development of inflammatory bowel disease (IBS)[17]. It has been reported that mast cells and their inflammatory mediators are closely associated with a number of intestinal diseases including idiopathic IBD. Parasites induce mast cell degranulation, release histamine. Mast cells from healthy controls don’t produce IL-5, but mast cells from patients with intestinal inflammatory disease could release a relatively large amount of IL-5[18,19]. IL-5 induced by parasites may play a role in causing IBD.
The aim of this study was to compare the IL-5 cytokine measurements in the cell cultures of patients who presented with allergic complaints and diarrhea and were positive for parasites in stool examinations with the IL-5 syntheses determined in serum samples, and to investigate eosinophilia observed in helminth and protozoon infections accompanied with allergy and diarrhea.
Twenty-three patients with intestinal parasites and 21 controls who had allergic complaints and not any intestinal parasite and diarrhea, were included in this study.
The study group included 12 females and 11 males, aged 18-45 and 25-53 years respectively. The control group included 9 females and 12 males, aged 20-45 and 28-50 years respectively.
IL-5 production in in vitro cell cultures prepared by using PHA to stimulate PBMC obtained from these patients was compared with the IL-5 level in serum. In addition, IL-5 production in protozoon and helminth infections was also compared.
PBMC and serum samples separated from 5 mL of heparinized blood samples from the patients using the Ficoll hypaque density gradient centrifuge method were utilized in the study.
PBMC (83 × 105/mL) was incubated with PHA in RPMI 1640 + 10% HuS inside 96 well tissue culture plates containing 5 mL/L CO2 for 48 h. Supernatants were taken at the end of 48 h and kept at -20 °C until cytokine analysis was carried out[20].
IL-5 levels in the supernatant and serum samples were evaluated using the double sandwich ELISA as previously described by Geiger et al[21].
Absolute values in 1 mm³ of blood were calculated by means of peripheral blood smear from the patients within the study as well as the controls.
Results were evaluated using the Student t test.
Helminths were detected in 15 (65.2%) and protozoons in 8 (34.8%) of the 23 patients (Table 1). All the patients had diarrheal symptoms.
| Parasites | Those with parasite infection | Mean serum IL-5 (pg/mL) | Mean PHA-IL-5 (ng/mL) | Eosinophil % |
| E. vermicularis | 9 | 15.5 | 383.4 | 8 |
| A. lumbricoides | 1 | 9.98 | 750 | 9 |
| H. nana | 1 | 13.61 | 620 | 8 |
| T. saginata | 4 | 12.9 | 652.5 | 6 |
| E. coli | 3 | 21.15 | 415 | 2.4 |
| I.butschlii | 2 | 15.61 | 297 | 8 |
| B.hominis | 1 | 17.27 | NA | 4.4 |
| G.intestinalis | 2 | 22.35 | 872.5 | 3 |
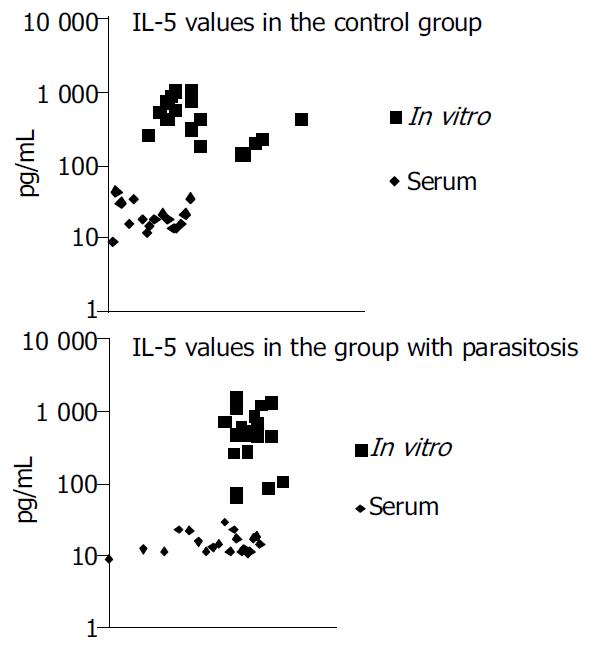
A comparison between the sera of the treated group and the control group did not reveal any significant differences (P > 0.05 and P > 0.05). The nonspecific IL-5 synthesis determined after stimulation with PHA was higher as compared to the IL-5 values measured in the serum (P < 0.05) (Figure 1).
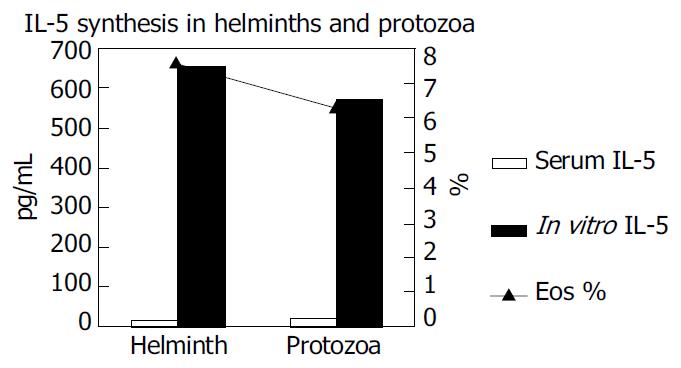
It was also established that the IL-5 cytokine measurements were higher in the group with protozoans than in the group with helminths. These values were also higher as compared to the cytokine measurements in the cell cultures (P < 0.05) (Figure 2).
The eosinophil rate was 7.0% in the study group whereas the eosinophil rate was 6.5% in the control group. However, significant difference could not be found between these two groups (P > 0.05) (Table 2).
| Serum IL-5 (pg/mL) | In-vitro IL-5 (pg/mL) | Eosinophil % | |
| Patients with parasite infection | 16.2 | 634 | 7.0 |
| Control | 32.4 | 885.4 | 6.5 |
Th2 response is important in protection against and destroying the parasites. Especially in some tropical areas, helminths were the major immediate triggers of Th2 response[22,23].
Pit et al[24] found that levels of IL-5 were induced by intestinal helminths. Protective immune responses to intestinal nematodes could be controlled by Th2 lymphocyte responses. Increased number of eosinophils in the GI mucosa and Th2 cell cytokine responsible for the generation of eosinophils, were prominent features of gastrointestinal nematode infections[25]. Herrstom et al[26] found that E. vermicularis were more frequently found in children with allergic disease as defined by allergic symptoms. Sorci et al[27] suggested that there was an association between the pinworms (oxyuridae, nematoda) and eosinophil concentration. Varga et al[28] reported that patients infected with A. lumbricoides, had more severe allergic symptoms, and allergic symptoms were seen in patients infected with G. intestinalis.
Previous studies, however, have shown that allergic complaints could precede other problems in protozoon infections. It was stated that allergic reactions also occurred in the disease caused by G. intestinalis that lives in the digestive tract. The relationship between allergy and giardiasis has been shown in various publications[1-29]. In Moscow, during the clinical studies of children with G. intestinalis infection, allergic reactions were found in 15.7%[30].
It was shown in some previous studies that IgG1, IgG3, IgM and IgA syntheses were important as resisting factors against the infection in intestinal protozoon infections such as giardiasis[31]. The presence of anti-parasitic antibodies detected in the serum depended on the secretion of cytokines such as IL-4 which is also produced from Th2 cells. As high antibody levels triggered by IL-4 in the serum, high IL-5 levels coming from the same source of cells were found to be higher than those from, supernatants in patients with protozoon infection.
It was also reported that urticaria and eosinophilia were detected in people infected with B. hominis[11]. Biedermann et al[32] reported that antigens from B. hominis caused urticaria. Hypersensitive (DTH) response to amebiosis delayed-type was evaluated in C3H/HeJ mice infected with E.histolytica[33].
It is important to measure and assess IL-5 as a cytokine triggering eosinophilia in patients who present with allergic complaints and whose stool examinations reveal no parasites. It was established in this study that the IL-5 synthesis determined in the serum samples in the group with protozoa was more significant than that determined in the cell cultures in the group with helminths. Thus, eosinophilia was not only limited to helminth infection, but also in protozoon infection.
Eosinophils account was consisted of 2%-3% of the total leukocytes in the blood and functioned as cytotoxic effector cells in allergic and parasitic disease, especially for the elimination of parasites[34,35]. Eosinophilia occurred due to the IL-5 effect that reflects Th2 cell secretion[36]. A number of studies have shown the presence of eosinophilia in parasitic infections. The blood eosinophils are associated with helminthic diseases. The primary function of the eosinophil is to protect against helminth parasites and eosinophils are responsible for a considerable amount of inflammatory pathology accompanying helminth infections[16-37].
In the present study, the rate of eosinophilia was 7% in the study group and 6.5% in the control group. Because of the control group also had allergic complaints, no statistically significant difference could be found between the two groups (P > 0.05).
It has also been shown that PBMC from healthy volunteers whose lymphocytes would not produce IL-5, failed to produce IL-5 after stimulation with PHA[38]. Consistent with the results of Katial et al[38], IL-5 values obtained for 16 healthy volunteers in the present study after stimulation with PHA were lower than those in the group with parasite infection and the control group.
Parasites induce IL-5 secretion. The effect of IL-5 on IBD should be investigated[18]. Approximately 25% of patients with IBS had a history of infectious enteritis. Microbial agents including parasites could increase the number of mast cells within the colonic muscle wall, release pro-inflammatory substances, and increase the number of inflammatory cells that might cause IBS[17].
Edited by Wang XL Proofread by Chen WW and Xu FM
| 1. | Giacometti A, Cirioni O, Antonicelli L, D'Amato G, Silvestri C, Del Prete MS, Scalise G. Prevalence of intestinal parasites among individuals with allergic skin diseases. J Parasitol. 2003;89:490-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Falcone FH, Loukas A, Quinnell RJ, Pritchard DI. The innate allergenicity of helminth parasites. Clin Rev Allergy Immunol. 2004;26:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Black P. Why is the prevalence of allergy and autoimmunity increasing? Trends Immunol. 2001;22:354-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Finkelman FD, Urban JF. The other side of the coin: the protective role of the TH2 cytokines. J Allergy Clin Immunol. 2001;107:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, Smith P, McKenzie AN. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 264] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Garside P, Kennedy MW, Wakelin D, Lawrence CE. Immunopathology of intestinal helminth infection. Parasite Immunol. 2000;22:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Holt PG. Parasites, atopy, and the hygiene hypothesis: resolu-tion of a paradox? The Lancet. 2000;18; 1699-1700. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Cribier B, Noacco G. [Chronic urticaria and infectious diseases]. Ann Dermatol Venereol. 2003;130 Spec No 1:1S43-1S52. [PubMed] |
| 10. | Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 819] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 11. | Barahona Rondón L, Maguiña Vargas C, Náquira Velarde C, Terashima I A, Tello R. [Human blastocystosis: prospective study symptomatology and associated epidemiological factors]. Rev Gastroenterol Peru. 2003;23:29-35. [PubMed] |
| 12. | Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Capron M, Dombrowicz D. [Eosinophils, parasites and allergy: from the biological to the clinical]. J Soc Biol. 2002;196:23-28. [PubMed] |
| 14. | Lawrence CE. Is there a common mechanism of gastrointestinal nematode expulsion? Parasite Immunol. 2003;25:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Levy DA. Parasites and allergy: from IgE to Th1/Th2 and beyond. Clin Rev Allergy Immunol. 2004;26:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309-318. [PubMed] |
| 19. | He SH, Xie H, He YS. Induction of tryptase and histamine release from human colon mast cells by IgE dependent or independent mechanisms. World J Gastroenterol. 2004;10:319-322. [PubMed] |
| 20. | Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect Immun. 2003;71:2422-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Geiger SM, Massara CL, Bethony J, Soboslay PT, Carvalho OS, Corrêa-Oliveira R. Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunol. 2002;24:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Turner JD, Faulkner H, Kamgno J, Cormont F, Van Snick J, Else KJ, Grencis RK, Behnke JM, Boussinesq M, Bradley JE. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis. 2003;188:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Pit DS, Polderman AM, Schulz-Key H, Soboslay PT. Prenatal immune priming with helminth infections: parasite-specific cellular reactivity and Th1 and Th2 cytokine responses in neonates. Allergy. 2000;55:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Onah DN, Nawa Y. Mucosal immunity against parasitic gastrointestinal nematodes. Korean J Parasitol. 2000;38:209-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Herrström P, Henricson KA, Råberg A, Karlsson A, Högstedt B. Allergic disease and the infestation of Enterobius vermicularis in Swedish children 4-10 years of age. J Investig Allergol Clin Immunol. 2001;11:157-160. [PubMed] |
| 27. | Sorci G, Skarstein F, Morand S, Hugot JP. Correlated evolution between host immunity and parasite life histories in primates and oxyurid parasites. Proc Biol Sci. 2003;270:2481-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Varga M, Dumitraşcu D, Piloff L, Chioreanu E. Skin manifestations in parasite infection. Roum Arch Microbiol Immunol. 2001;60:359-369. [PubMed] |
| 29. | Di Prisco MC, Hagel I, Lynch NR, Jiménez JC, Rojas R, Gil M, Mata E. Association between giardiasis and allergy. Ann Allergy Asthma Immunol. 1998;81:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Zalipaeva TL. [Clinical symptoms of giardia infection in children]. Med Parazitol (Mosk). 2002;29-32. [PubMed] |
| 31. | Soliman MM, Taghi-Kilani R, Abou-Shady AF, El-Mageid SA, Handousa AA, Hegazi MM, Belosevic M. Comparison of serum antibody responses to Giardia lamblia of symptomatic and asymptomatic patients. Am J Trop Med Hyg. 1998;58:232-239. [PubMed] |
| 32. | Biedermann T, Hartmann K, Sing A, Przybilla B. Hypersensitivity to non-steroidal anti-inflammatory drugs and chronic urticaria cured by treatment of Blastocystis hominis infection. Br J Dermatol. 2002;146:1113-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Ghosh PK, Gupta S, Ortiz-Ortiz L. Intestinal amoebiasis: delayed-type hypersensitivity response in mice. J Health Popul Nutr. 2000;18:109-114. [PubMed] |
| 34. | Meeusen EN, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Dombrowicz D, Capron M. Eosinophils, allergy and parasites. Curr Opin Immunol. 2001;13:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Zacharia BE, Sherman P. Atopy, helminths, and cancer. Med Hypotheses. 2003;60:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Schneider M, Hilgers RH, Sennekamp J. Allergy, total IgE and eosinophils in East and West -- serious effects of different degrees of helminthiasis and smoking. Eur J Med Res. 2002;7:63-71. [PubMed] |
| 38. | Katial RK, Sachanandani D, Pinney C, Lieberman MM. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin Diagn Lab Immunol. 1998;5:78-81. [PubMed] |