Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3612
Revised: April 22, 2004
Accepted: April 29, 2004
Published online: December 15, 2004
AIM: To investigate the inhibitory effects of sodium orthovanadate on small-intestinal glucose and maltose absorption in rats and its mechanism.
METHODS: Normal Wistar rats were lavaged with sodium orthovanadate (16 mg/kg, 4 mg/kg and 1 mg/kg) for 6 d. Blood glucose values were measured after fasting and 0.5, 1, 1.5 and 2 h after glucose and maltose feeding with oxidation-enzyme method. α-glucosidase was abstracted from the upper small intestine, and its activity was examined. mRNA expression of α-glucosidase and glucose-transporter 2 (GLUT2) in epithelial cells of the small intestine was observed by in situ hybridization.
RESULTS: Sodium orthovanadate could delay the increase of plasma glucose concentration after glucose and maltose loading, area under curve (AUC) in these groups was lower than that in control group. Sodium orthovanadate at dosages of 10 μmol/L, 100 μmol/L and 1000 μmol/L could suppress the activity of α-glucosidase in the small intestine of normal rats, with an inhibition rate of 68.18%, 87.22% and 91.91%, respectively. Sodium orthovanadate reduced mRNA expression of α-glucosidase and GLUT2 in epithelial cells of small intestine.
CONCLUSION: Sodium orthovanadate can reduce and delay the absorption of glucose and maltose. The mechanism may be that it can inhibit the activity and mRNA expression of α-glucosidase, as well as mRNA expression of GLUT2 in small intestine.
- Citation: Ai J, Du J, Wang N, Du ZM, Yang BF. Inhibition of small-intestinal sugar absorption mediated by sodium orthovanadate Na3VO4 in rats and its mechanisms. World J Gastroenterol 2004; 10(24): 3612-3615
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3612.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3612
There is evidence that sodium orthovanadate could markedly decrease the high blood sugar induced by alloxan and streptozotocin[1,2]. Sodium orthovanadate acts on blood sugar by increasing the number of insulin-receptors and enhancing glucose transport via promoting the combination of insulin and insulin receptors[3-7]. Besides, sodium orthovanadate can also inhibit glucose absorption from small intestines of rats by inhibiting the activity of Na+/K+-ATPase and increasing contractility in the intestinal smooth muscles[8,9]. Therefore we observed the effects of sodium orthovanadate on glucose and maltose absorption in the small intestine of Wistar rats and investigated the mechanisms in order to provide theoretical basis for its further development.
Male and female Wistar rats weighing 180-220 g were obtained (Department of Animals, Harbin Medical University). Sodium orthovanadate was provided by Harbin Medical University. Acarbose was purchased from Bioer (Wuhan, China). Both α-amylase and ρ-nitrobenzene-α-D-malt pentose glycoside were supplied by Sigma (America). In situ hybridization kits of the small intestine GLUT2 and α-glucosidase were from BOSD Biotech (Wuhan, China).
Experimental schedule Normal Wistar rats were randomly divided into 5 groups (8 rats per group). Animals in group 1 were lavaged with saline, rats in groups 2-5 were lavaged with acarbose (30 mg/kg) and sodium orthovanadate (16 mg/kg, 4 mg/kg and 1 mg/kg ) for 6 d at the dosage 1 mL/100 g. Blood glucose values 12 h after fasting and 0.5, 1, 1.5 and 2 h after feeding glucose (22 g/kg) were investigated using oxidation-enzyme method. The same method was used to determine the effects of sodium orthovanadate on maltose absorption. Small-intestine tissue was obtained after the blood glucose assay for in situ hybridization analysis.
α-glucosidase activity assay As described previously[10,11], normal rats were killed 3 h after fasting. A 10 cm segment of the upper small intestine from the head of the dodecadactylon was washed twice with cool saline. Mucosae were homogenized after diluted at 1:10 in 0.5 mol/L NaCl-KCl buffer, then centrifuged at 2 × 104 g for 30 min (4 °C). Deposits were washed twice with cool saline, then 2 × 104 g centrifuged for 30 min (4 °C), diluted at 1:5 in saline again and 500 r/min centrifuged for 10 min (4 °C). Supernatant was collected and stored at -30 °C. After response architecture was dispensed, fluid samples were shaken and water-bathed for 10 min at 30 °C. Then samples were put into water bath at 85 °C to terminate the response. In the response architecture without enzymes and drugs, the control group was adjusted to zero absorption degree (A). In the response architecture without drugs, the standard group’s enzyme activity was 100%. A was assayed at 405 nm wavelength. Then we calculated the percentage of inhibition of 10 μmol/L, 100 μmol/L and 1000 μmol/L sodium orthovanadate on α-glucosidase activity.
In situ hybridization for α-glucosidase and GLUT2 expression A 2 cm segment of the upper small intestine was obtained from the head of dodecadactylon. In situ hybridization was carried out according to the manual of BOSD Biotech. Two microliters thick specimens sectioned from a parafin-embedded block were dewaxed in xylene and rehydrated in serially graded ethanol (100%, 95%). The activity of endogenous enzymes was deactivated by hydrogen peroxide solution (30%) for 1 min. Specimens were ingested with pepsin for 15 min at 20-22 °C, force-hybridized for 3 h at 38 °C and hybridized for 12 h at 38 °C. Finally, the slides were washed 4 times with PBS, each time for 5 min. After trickled with biotin-rat antibodies to digoxin at 37 °C for 60 min, then streptavidin-biotin-enzyme complex (SABC) was added at 37 °C for 20 min and biotin-peroxidase was added at 37 °C for 20 min. At last the slides were washed 4 times with PBS, each time for 5 min, and stained with DAB for 2-4 min. The positive expression of α-glucosidase showed brown staining signals in villi of small intestine and GLUT2 in incisures between two villi of small intestine.
Data was tested using Student’s t-test. The measurement results were expressed as mean ± SD.
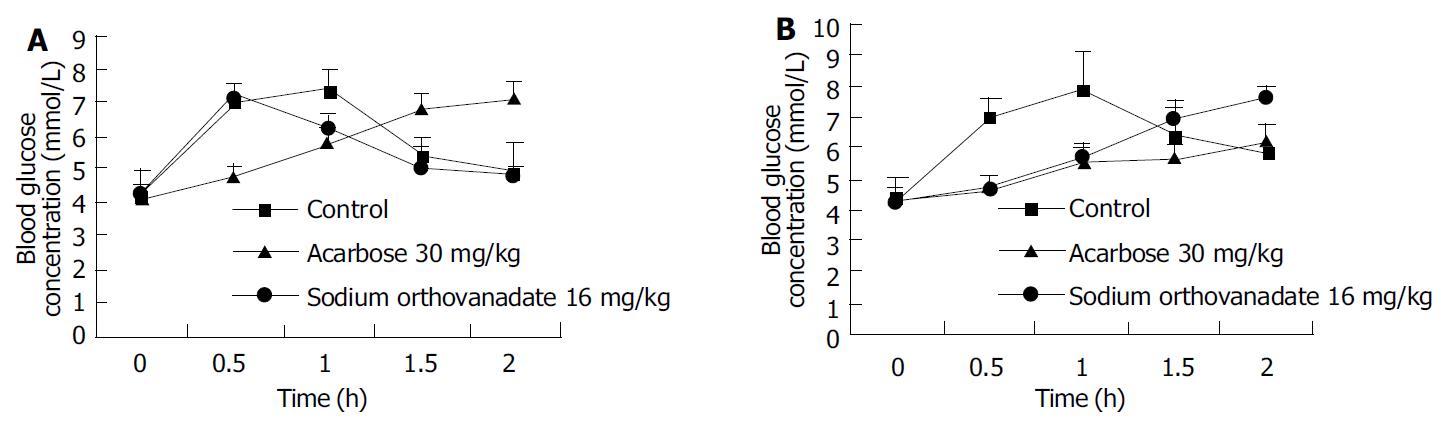
Sodium orthovanadate at the dosage of 10 µmol/L, 100 µmol/L, 1000 µmol/L delayed the increase of blood glucose concentration induced by lavaged glucose (22 g/kg) after 1.5-2 h (Figure 1A), and decreased the AUC in these groups to that in control [17.03 ± 0.60mmol/(L·h)](P < 0.05),whichwas[8.24 ± 0.63mmol/(L·h)] (P < 0.01), [9.69 ± 0.38 mmol/(L·h)] (P < 0.01), [13.76 ± 0.39 mmol/(L·h)] (P < 0.05), respectively.
Blood glucose concentration increase in rats after maltose feeding (22 g/kg) was delayed by both sodium orthovanadate and acarbose, the peak values were shown 2 h after maltose loading, and high and moderate dosages of sodium orthovanadate also inhibited the increase of the blood glucose concentration peak value (P < 0.05) (Figure 1B). The AUC of blood glucose concentration in sodium orthovanadate groups at the 3 dosages was markedly lower than that in the control group [18.40 ± 1.46 mmol/(L·h)]. AUC at high and moderate dosages was [8.97 ± 1.56 mmol/(L·h)], and [6.19 ± 0.47 mmol/(L·h)], both were lower than that in acarbose group with AUC [13.10 ± 0.43 mmol/(L·h)] (P < 0.05).
Sodium orthovanadate at the dosages of 10 µmol/L, 100 µmol/L, 1000 µmol/L, could inhibit α-glucosidase activity in small intestine, the percentage of inhibition was 68.18%, 87.22% and 91.91%, respectively. The inhibiting action of sodium orthovanadate (100 µmol/L) was stronger than that of the same dosage of acarbose (70.37%) (P < 0.05, Table 1).
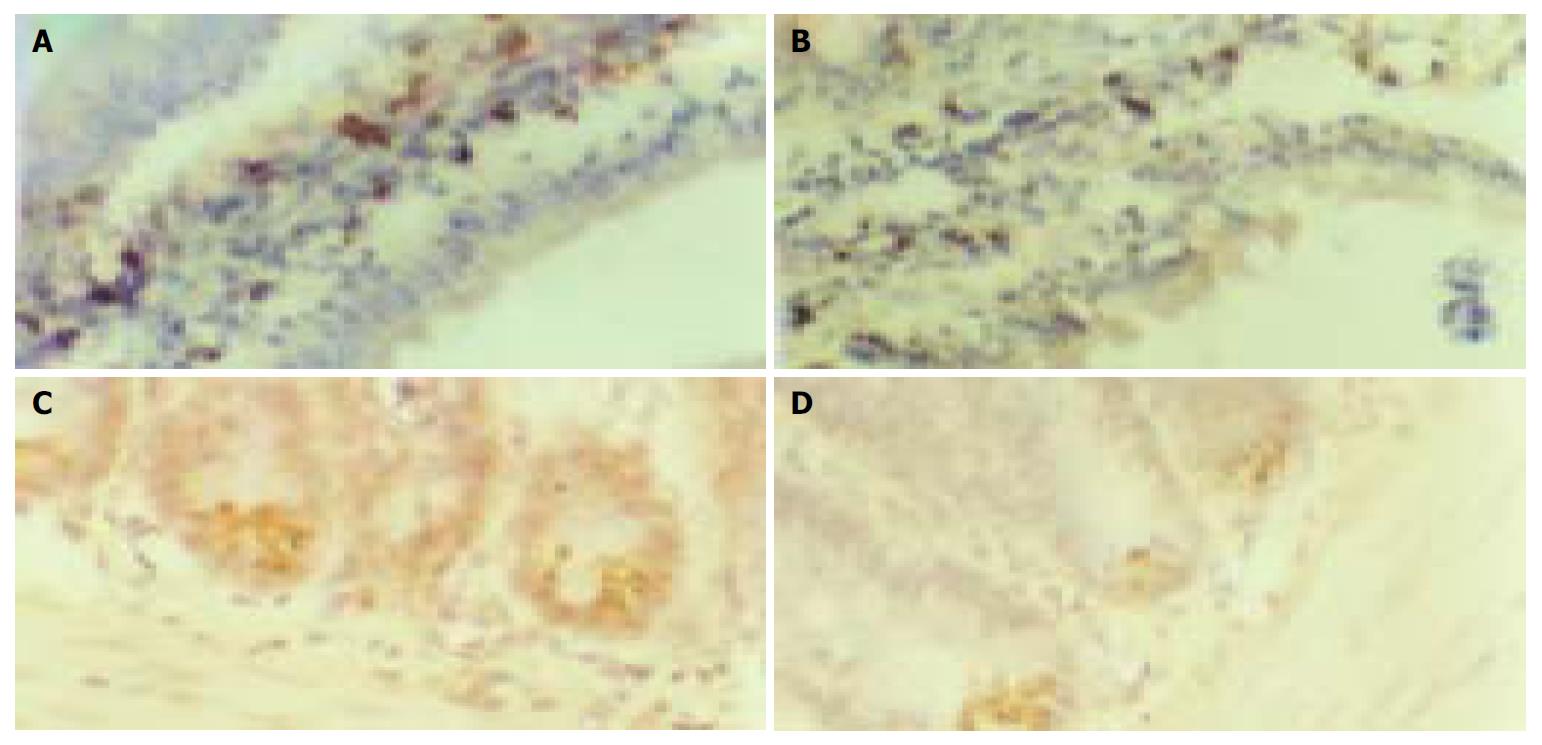
Sodium orthovanadate at the dosages of 1 mg/kg, 4 mg/kg and 16 mg/kg, could depress α-glucosidase mRNA expression in epithelial cells of small-intestine, the positive expression number of α-glucosidase particles in each villus was 38 ± 5, 34 ± 5 and 33 ± 4. They were fewer than that in control group (45 ± 6), (P < 0.05) (n = 16) (Figure 2A, B). The inhibiting action of sodium orthovanadate showed no marked difference between high and moderate dosages. GLUT2 mRNA was diffusely expressed in the incisures between two villi of small intestine. Sodium orthovanadate could inhibit GLUT2 mRNA expression in a dose-dependent manner. In the vision field of 100 cm2, the expression area of GLUT2 mRNA was decreased to 0.5 ± 0.12 cm 2, 0.77 ± 0.15 cm 2, 1.02 ± 0.24 cm 2 after sodium orthovanadate loading at the 3 dosages from 2.5 ± 0.5 cm 2 in control group (P < 0.05) (n = 16) (Figure 2C, D).
First, in this study, we observed the effects of sodium orthovanadate on glucose and maltose absorption. The result proved that sodium orthovanadate could delay the increase of blood glucose concentration after glucose or maltose feeding and decrease AUC.
Generally, sugar is divided into monosaccharide, disaccharide and polysaccharide. The absorption of monosaccharide is an active transport process with energy consumption. Na+-dependent transporters exist in the brush border of epithelial cells in intestinal mucosae, which can transport Na+ along its concentration gradient and monosaccharide counter to its concentration gradient into cells from intestinal lumen via the brush border, then both Na+ and monosaccharide diffuse into blood. In this process, the exchange activity of Na+/K+-ATPase plays an important role in maintaining the Na+ concentration gradient. Decreased activity of Na+/K+-ATPase can influence glucose transport and therefore decrease blood glucose concentration. In addition, GLUT2 existing in the epithelial cells of intestinal villi incisures can directly transport glucose into cells. Decreased expression and limited translocation process from cytoplasm to cell membranes of GLUT2 will decrease glucose absorption from the small intestine. In normal conditions, polysaccharides are the mainly existing pattern in foods, which can be absorbed into blood after degradation into oligosaccharides and disaccharides by amylase and further into monosaccharides by α-glucosidase existing in the brush border of epithelial cells in intestinal mucosae. So α-glucosidase is a very important factor, which can affect glucose absorption in small intestine. Acarbose, a kind of inhibitors of α-glucosidase used in clinic at present, can depress the absorption of polysaccharides by inhibiting α - glucosidase activity and effectively decrease postprandial blood glucose level of diabetes mellitus[12,13], therefore Na+/K+-ATPase, GLUT2 and α-glucosidase play the main role in increasing postprandial blood glucose concentration. Postprandial blood glucose concentration would change if any of them has dysfunction.
Sodium orthovanadate has antihyperglycemic actions by various means, such as increasing the protein expression of GLUT4 in skeletal muscles, accelerating hexose transport, increasing glycogen synthetase production by stimulating dextrose oxidan[14,15], accelerating glycolysis and lipid synthesis, depressing Na+ reabsorption via acting on Na+/Ca2+ exchanger[16].
In the present study, we only investigated the effects of sodium orthovanadate on small-intestinal sugar absorption in rats. First, we observed the effects of sodium orthovanadate on glucose absorption. Glucose is a monosaccharide, which can be directly absorbed by the small-intestine without hydrolysis, so the function of both GLUT2 and Na+/K+-ATPase can be reflected by its absorption state. The first body response after monosaccharide absorption is to induce insulin release by stimulating pancreatic islets to decrease blood glucose concentration. For this reason, blood sugar peak values show the tendency to elevate first and then decline, thus typical OGTT curve is formed. Studies have shown that acarbose, a kind of inhibitors of α-glucosidase, can inhibit the hydrolysis of disaccharides and polysaccharides, and then decrease postprandial blood glucose concentration and protect OGTT, but it could not depress glucose absorption. Our results are similar to previous studies[17-19]. In order to eliminate the lowering effect on blood sugar of insulin induced by pancreatic islets, sodium orthovanadate was orally administered into rats for 6 d to maintain a stabilizing effect on islet cells. Our results showed that sodium orthovanadate at all the 3 dosages could delay the absorption of glucose and blood sugar concentration peak value with decreased AUC in a dose-dependent manner. Compared with the control group, blood sugar concentration decrease at 0.5 h and 1 h after sodium orthovanadate feeding was due to decreased glucose absorption, but not due to insulin release, because AUC peak value was delayed but not extincted. The results imply that the activity and function of both Na+/K+-ATP and GLUT2 may participate in this kind of actions of sodium orthovanadate. Studies have evidenced that sodium orthovanadate is a Na+/K+-ATPase inhibitor, and has been used widely as a tool drug[20]. So it is generally accepted that sodium orthovanadate can depress sugar absorption via inhibiting the activity of Na+/K+-ATPase in the small intestine. The results in our study suggest that sodium orthovanadate can inhibit sugar absorption via affecting the function of GLUT2, because it could markedly inhibit the expression of GLUT2 mRNA in small-intestinal epithelia of normal rats.
But we still do not know if it is the whole mechanism of sodium orthovanadate underlying the inhibition of sugar absorption, and whether it also can affect the absorption of polysaccharides and disaccharides. Therefore we estimated the effect of sodium orthovanadate on maltose absorption.
Maltose is composed of two monosaccharides coupled with α-1,4 glycoside linkages, which can be absorbed only after it is hydrolyzed by α-glucosidase in the intestinal tract. The data reported here suggest that sodium orthovanadate can delay the absorption of maltose and the blood sugar concentration peak value in a dose-dependent manner. Moreover, the AUC of sodium orthovanadate in moderate and large dosage groups and acarbose group was definitely lower than that in the control group. The action of sodium orthovanadate was stronger than that of acarbose, because the AUC of sodium orthovanadate in moderate and high dosage groups was lower than that in the acarbose group. This phenomenon may be due to two reasons: One is the inhibition of sodium orthovanadate on the activity of Na+/K+-ATPase and GLUT2 mRNA expression was stronger than that of acarbose on α-glucosidase, the other is that sodium orthovanadate might also inhibit the activity of α-glucosidase besides its action on Na+/K+-ATPase and GLUT2 mRNA expression. Its double action decreased blood sugar markedly. In order to further verify our tentative idea, we observed the influence of sodium orthovanadate on the activity of α-glucosidase; the results indicated that sodium orthovanadate could depress α-glucosidase activity in a dose-dependent manner.
There are two ways of sodium orthovanadate in decreasing the activity of α-glucosidase. One is that sodium orthovanadate could decrease α-glucosidase activity without enzyme protein content changes. The other is that it could reduce the expression of α-glucosidase. So we detected mRNA expression of α-glucosidase by in situ hybridization. The result demonstrated that sodium orthovanadate could depress the mRNA expression of α-glucosidase in a dose-dependent manner.
In conclusion, sodium orthovanadate has the ability to inhibit sugar absorption via versatile means which affect the function and activity of Na+/K+-ATPase, inhibit the activity and gene expression of α-glucosidase as well as depress gene expression of the glucose transporter.
Co-correspondents: Jing Ai
Edited by Wang XL and Zhu LH Proofread by Xu FM
| 1. | Meyerovitch J, Farfel Z, Sack J, Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J Biol Chem. 1987;262:6658-6662. [PubMed] |
| 2. | Meyerovitch J, Waner T, Sack J, Kopolovic J, Shemer J. Attempt to prevent the development of diabetes in non-obese diabetic mice by oral vanadate administration. Isr Med Assoc J. 2000;2:211-214. [PubMed] |
| 3. | Shao J, Catalano PM, Yamashita H, Ishizuka T, Friedman JE. Vanadate enhances but does not normalize glucose transport and insulin receptor phosphorylation in skeletal muscle from obese women with gestational diabetes mellitus. Am J Obstet Gynecol. 2000;183:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (2)] |
| 4. | Marita AR, Anilkumar KL. Effect of vanadate on glycogen synthesis in dexamethasone-treated 3T3 adipocytes: evidence for a novel insulin sensitizing action. Diabetes Obes Metab. 2001;3:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Pugazhenthi S, Khandelwal RL, Angel JF. Insulin-like effect of vanadate on malic enzyme and glucose-6-phosphate dehydrogenase activities in streptozotocin-induced diabetic rat liver. Biochim Biophys Acta. 1991;1083:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Madsen KL, Ariano D, Fedorak RN. Vanadate treatment rapidly improves glucose transport and activates 6-phosphofructo-1-kinase in diabetic rat intestine. Diabetologia. 1995;38:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | O'Connor JC, Freund GG. Vanadate and rapamycin synergistically enhance insulin-stimulated glucose uptake. Metabolism. 2003;52:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kellett GL, Barker ED. The effect of vanadate on glucose transport and metabolism in rat small intestine. Biochim Biophys Acta. 1989;979:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 9. | Hudgins PM, Bond GH. Alteration by vanadate of contractility in vascular and intestinal smooth muscle preparations. Pharmacology. 1981;23:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Yasuda K, Shimowada K, Uno M, Odaka H, Adachi T, Shihara N, Suzuki N, Tamon A, Nagashima K, Hosokawa M. Long-term therapeutic effects of voglibose, a potent intestinal alpha-glucosidase inhibitor, in spontaneous diabetic GK rats. Diabetes Res Clin Pract. 2003;59:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wehmeier UF, Piepersberg W. Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose. Appl Microbiol Biotechnol. 2004;63:613-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Ron Y, Wainstein J, Leibovitz A, Monastirsky N, Habot B, Avni Y, Segal R. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci. 2002;57:M111-M114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Gupta D, Raju J, Prakash J, Baquer NZ. Change in the lipid profile, lipogenic and related enzymes in the livers of experimental diabetic rats: effect of insulin and vanadate. Diabetes Res Clin Pract. 1999;46:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Goldwaser I, Gefel D, Gershonov E, Fridkin M, Shechter Y. Insulin-like effects of vanadium: basic and clinical implications. J Inorg Biochem. 2000;80:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Rowe WA, Tomicic TK, Hajjar JJ. Enhancement of rat intestinal calcium absorption by vanadate. Proc Soc Exp Biol Med. 1991;198:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Bischoff H. Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest. 1994;24 Suppl 3:3-10. [PubMed] |
| 18. | Ranganath L, Norris F, Morgan L, Wright J, Marks V. Delayed gastric emptying occurs following acarbose administration and is a further mechanism for its anti-hyperglycaemic effect. Diabet Med. 1998;15:120-124. [PubMed] [DOI] [Full Text] |
| 19. | Joubert PH, Venter HL, Foukaridis GN. The effect of miglitol and acarbose after an oral glucose load: a novel hypoglycaemic mechanism? Br J Clin Pharmacol. 1990;30:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Scheiner-Bobis G, Hübschle T, Diener M. Action of palytoxin on apical H+/K+-ATPase in rat colon. Eur J Biochem. 2002;269:3905-3911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |