Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3559
Revised: January 5, 2004
Accepted: January 12, 2004
Published online: December 15, 2004
AIM: To investigate p53 mutation and p21 expression in hepatocarcinogenesis induced by hepatitis B virus (HBV) and aflatoxin B1 (AFB1) in tree shrews, and to reveal the role of these genes in hepatocarcinogenesis.
METHODS: Tree shrews were divided into four groups: group A, those infected with HBV and fed with AFB1 (n = 39); group B, those infected with HBV alone (n = 28); group C, those fed with AFB1 alone (n = 29); and group D, normal controls (n = 20). The tree shrews underwent liver biopsies once every 15 wk. Expression of p53 and p21 proteins and genes in the biopsies and tumor tissues of the experimental tree shrews was detected, respectively, by immunohistochemistry, and by Southern blotting and reverse transcription-polymerase chain reaction and sequencing.
RESULTS: The incidence of hepatocellular carcinomas (HCC) was higher in group A (66.7%) than that in group B (3.57%) and C (30%). The time of HCC occurrence was also earlier in group A than that in group C (120.0 ± 16.6 wk vs 153.3 ± 5.8 wk, respectively, P < 0.01). p53 protein was not detected by immunohistochemistry in all groups before the 75th wk of the experiment. At the 105th wk, the positive rates fo p53 were 78.6%, 60% and 71.4% in groups A, B and C, respectively, which were significantly higher than that in group D (10%) (all P < 0.05). An abnormal band of p53 gene was observed in groups A and C. The mutation points of p53 gene in tree shrews with HCC were at codons 275, 78 and 13. The nucleotide sequence and amino acid sequence of tree shrew’s wild-type p53 showed 91.7% and 93.4% homologies with those of human p53, respectively. The immunopositivity for p21 was found before HCC development. The incidence of HCC was significantly higher in tree shrews that were positive for p21 than those negative for p21 (80.0% vs 11.0%, P < 0.001). The incidence of HCC in p21 positive animals in group A was significantly higher than those positive for p21 in group C (P < 0.05).
CONCLUSION: A remarkable synergistic effect on HCC development exists between HBV and AFB1. p53 mutation promotes the development of HCC. HBV and AFB1 may synergistically induce p53 gene mutation, and stimulate ras gene expression. ras gene is activated at the earlier stage during hepatocarcinogenesis. p21 protein may be an early marker, and the alterations of p53 may be a late event in the development of HCC.
-
Citation: Su JJ, Ban KC, Li Y, Qin LL, Wang HY, Yang C, Ou C, Duan XX, Lee YL, Yang RQ. Alteration of
p53 andp21 during hepatocarcinogenesis in tree shrews. World J Gastroenterol 2004; 10(24): 3559-3563 - URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3559
Hepatocellular carcinoma (HCC) is one of the world’s most common cancers and is predominant in Africa and South-east Asia[1]. Epidemiological studies indicated that contamination of food with aflatoxin B1 (AFB1) and chronic infection with hepatitis B virus (HBV) are the major risk factors for human HCC[2,3].
p53, a tumor suppressor gene located on the short arm of chromosome 17, normally regulates the activity of the cell cycle machinery. Mutation of the p53 gene has been observed with a high prevalence in diverse types of human cancer and frequently occurs with point mutation. The frequencies of p53 mutation in HCC from different studies were varied from 18% to 67%[4,5]. Mutation of G to T transversion at the third base of codon 249 of p53 gene has been found in human HCC associated with high exposures to AFB1 in Africa and Qidong, China[6,7], Where this mutation is absent from HCC in the area with negligible exposure to AFB1. The ras gene coding for Mr 21000 protein (p21) binds guanine nucleotides and possesses GTPase activity. Through this mechanism, the ras p21 participates in the control of cell proliferation, possibly as a signal transducer from cell surface receptors to the nucleus[8]. H-ras oncogene could induce the metastatic phenotype of HCC cell in vitro to enhance its metastatic potential. The mutations of ras genes at codons 12, 13, and 61 leading to the increased expression of normal or mutant form of the p21 protein have been observed in human HCC and several other tumors[9]. However, some report that ras proto-oncogene can enhance or inhibit the malignant phenotype in vivo in different systems[10]. In the present study, using tree shrew (Tupaia belangeri chinensis) as an animal model for studying the development of HCC induced by human HBV and/or AFB1, we investigated the alterations of p53 and p21 during hepatocarcinogenesis in tree shrews.
Tree shrews were obtained from Kunming Medical Biology Institute, Chinese Academy of Sciences. One hundred and sixteen adult tree shrews weighed 127 ± 14.5 g. Animals were raised in stainless steel cage individually at room temperature of 25 ± 1 °C and fed with basic diet supplemented with fruits, milk, and eggs. Drinking water was given ad libitum.
Sera positive for HBV surface antigen (HBsAg), e antigen (HBeAg) and antibody against c antigen (anti-HBc) were obtained from several blood donors. The titres of HBsAg and HBeAg were more than 1:1024. The sera were preserved in a refrigerator at -40 °C and pooled before inoculation.
AFB1 was purchased from Sigma Chemical Co., USA. It was dissolved into dimethylsulphoxide (DMSO) and mixed with milk to be sipped by tree shrews.
Rabbit polyclonal antibody against human p53 protein (CM1) and avidin-biotin peroxidase complex kit were purchased from Vector Laboratories Inc., USA. RNase mini-kit was purchased from Qiagen Inc., Germany. PCR kit was the product of Stratagene Inc., USA. Rat antibody against human p21 (pan-ras) was purchased from Biosource Inc., USA.
Blood sample 1 mL was drawn through the femoral veins of each tree shrew before the experiment started. Some tree shrews were inoculated with 0.5 mL of human HBV-infected serum via the femoral vein. Three days later, another 0.5 mL of the same serum was injected peritoneally. After a week, the sera of these animals were checked weekly for HBV infection markers by enzyme-linked immunosorbent assay (ELISA). HBV- infected tree shrews confirmed by ELISA were randomly divided into group A (39 animals) and group B (28 animals). The un-inoculated tree shrews were randomly divided into group C (29 animals) and group D (20 animals). The animals of groups A and C were given AFB1, 200-400 µg/kg.b.m. per day, while group D was used as control. Liver biopsies were performed in each group once every 15 wk. The samples of liver biopsy or HCC were cut into 2 pieces. One was fixed in 40 g/L buffered formaldehyde, and the other was kept at -80 °C after immersed in liquid nitrogen.
Three samples of liver biopsy and 5 samples of HCC tissues stored at -80 °C were sent to Korea Research Institute of Bioscience and Biotechnology for Southern blotting to determine the p53 gene status.
Total RNAs were extracted from 10 mg of frozen tumors or biopsied liver tissues. Exon 2-4 (415 bp) of the p53 gene was amplified using the forward primer CDF2: 5’-ATTGGCAGCCA GACTGCCTTCCGGG-3’ and reverse primer CDR4: 5’-CGATT CTAGAGCAAAACATCTTGTTGAGGG-3’. Exon 5-11 (974 bp) of the p53 gene was amplified using the forward primer CDF5: 5’-CGATGAATTCTTGCATTCTGGGACAGCCAA-3’, and reverse primer CDR11: 5’-CGATAAGCTTCTGACGCACACCT ATTGCAA-3’. The reactions containing 0.5 µg total RNA, 5 mmol/L MgCl2, 1 mmol/L dNTP, 20 pmoL of each primer, 40 units of RNase inhibitor, 5 units of AMV reverse transcriptase, 5 units of AMV Tag DNA polymerase in a final volume of 50 µL in mRNA selective PCR buffer were used. The mixture of reactants was incubated at 42 °C for 30 min followed by PCR amplification with 25 cycles of at 85 °C for 30 s and at 72 °C for 1 min. PCR products were analyzed on 15 g/L agarose gel containing ethidium bromide. The PCR product of p53 was purified and sequenced by an automatic DNA sequencer.
p53 and p21 proteins were detected by immunohistochemical staining using the avidin-biotin complex (ABC) method on the sections of liver tissues and tumors. In brief, formalin-fixed, paraffin-embedded sections were deparaffinized in xylene and were passed through ethanol series. After the endogenous peroxidase activity was blocked, the sections were rinsed in 0.01 mol/L PBS. Non-specific binding was blocked by treatment with 5% normal horse or goat serum for 20 min. Primary antibody was applied to the sections and incubated in a moist chamber overnight at 4 °C. After the sections were washed in 0.01 mol/L PBS, biotinylated horse or goat anti-mouse or rabbit immunoglobulin G was applied and sections were incubated for 50 min at room temperature. After washed, the sections were incubated with avidin-biotin-peroxidase complex for 50 min and than washed again. The chromogen, 3-3’-diaminobenzidine (DAB) was added for 5 to 10 min. Finally, the sections were washed and mounted. The section without primary antibody served as negative control.
Differences of HCC incidence and percentages of immunopositivity for p53 and p21 were analyzed by the Chi-square (χ2) test. The difference of the average time for appearance of HCC was analyzed by the Student’s t test.
The first case of HCC appeared at the 83rd wk of the experiment in group B. At that time the number of living animals in groups A, B, C and D were 39, 28, 29 and 20, respectively. After feeding of AFB1 for 105 wk, the amount of AFB1 in groups A and C was 11.6-15.5 mg and 11.4-16.09 mg, respectively. The difference was not statistically significant. The experimental period was 160 wk.
HCC occurred only in groups A, B and C, and the rates of HCC in those groups were 58.9% (23/39), 3.57% (1/28) and 20.68% (6/29), respectively. No HCC was observed in group D. One tree shrew died at the 120th wk in group B had two proliferating pale nodules with 0.5 cm in diameter. They were large proliferating nodules of liver cells under the microscope. Not only was the HCC incidence higher but also the average time for HCC appearance was significantly shorter in group A than that in group C (120 ± 16.6 wk vs 153 ± 5.8 wk, t = 3.336, P < 0.01).
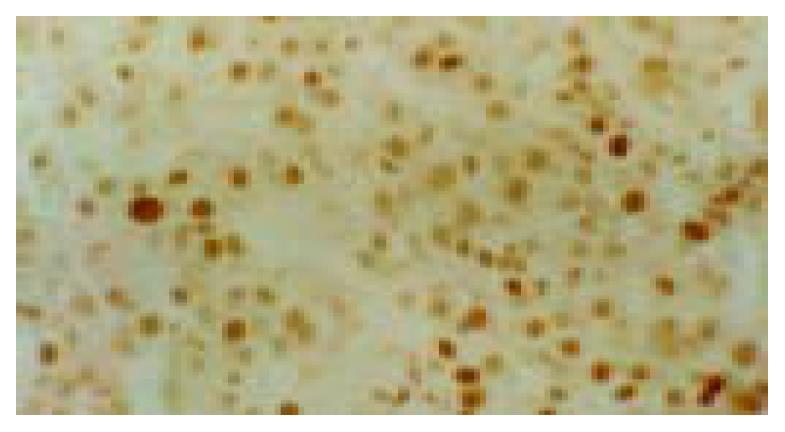
No p53 protein was detected by immunohistochemistry in each group at the 75th wk. The percentages of immunopositivity for p53 in group A (78.6%), B (60%) and C (71.4%) were significantly higher than that in group D (10.0%) at the 105th wk. The positive rates for p53 in HCC samples were not significantly different between groups A and C (52.17%, 12/23 vs 50%, 3/6, P > 0.05). Immunopositive signal of p53 was located in nucleus of cell (Figure 1). At the 105th wk, the extra bands of p53 were detected in 2 of 3 liver biopsy tissues from group C but none from other groups by Southern blotting. One case from group C appeared a new band with a size of 12.8 kb and lost a normal band of 1.6 kb at the same time. This tree shrew developed HCC at the 120th wk. A new band of 3.4 kb was found in another case from group C. Before the experiment started and during the 15th to 75th wk of the experiment, totally 12 biopsy liver tissues from 2 animals in group C were detected by Southern blotting and a light extra band of 3.8 kb was found in the tissues of 45th wk. Two of 8 HCCs from group A also appeared an extra band of 4.8 kb. The point mutation of p53 gene from 3 of 8 tumors was found by sequencing. The mutations were at codons 275, 78 and 13 (changing proline to serine, T→C transversion at the first nucleotide of codon 275; changing cystein to arginine, A→G transversion at the third position of codon 78; C→T transversion at the second position of codon 13; no amino acid change) (Table 1). The nucleotide sequence and amino acid sequence of tree shrew’s wild-type p53 showed 91.7% and 93.4% homologies with those of human p53 sequence, and 77.2% and 73.4% homologies with mouse p53 sequence, respectively[11].
| Animal (Groups) | Treatment | Tissues | Grade of tumors | Status of p53 gene | Change of nucleic acid | |
| HBV | AFB1 | |||||
| 5201 (C) | - | + | liver | - | wt | - |
| 5207 (C) | - | + | tumor | I | wt | - |
| 5063 (A) | + | + | tumor | II | mt (78) | GCA-GCG |
| 5067 (A) | + | + | tumor | II | wt | - |
| 5172 (A) | + | + | tumor | III | mt (13) | CCT-CTT |
| 5173 (A) | + | + | tumor | II | mt (275) | TGT-CGT |
| 1 (D) | - | - | liver | - | wt | - |
| 2 (D) | - | - | liver | - | wt | - |
p21 protein was located in plasma of liver cells (Figure 2) and overexpressed in totally 16 animals’ liver tissues in group A during hepatocarcinogenesis. All those tree shrews developed HCC. However, only 7 of 23 tree shrews were p21-negative in liver tissues, and developed HCC in the same group at the end of experiment. The incidence of HCC was significantly higher in these p21-positive animals than in those negative ones. There were 2 animals positive for p21 protein in the liver tissues in group B, one of them died at the 120th wk and had 2 proliferating nodules with 0.5 cm in diameter. They were large proliferating nodules of liver cells under microscope. At the end of the experiment, 4 of 7 tree shrews that were positive for p21 developed HCC in Group C, whereas only 2 of 22 tree shrews that were negative for p21 developed HCC in this group. p21 overexpression was not found in group D (Table 2). Totally 25 animals were positive for p21 in liver tissues in all groups and 20 of them developed HCC (80%), while only 10 of totally 91 animals which were negative for p21 developed HCC (10.99%). Only 1 sample of HCC tissue was positive for p21 in all 30 samples of HCC tissues (Table 3).
Animals in groups A and B were verified for infection with human HBV[12]. In the present study, not only was the incidence of HCC significantly higher but also the average time for HCC development was shorter in the animals both infected with HBV and exposed to AFB1 than those infected with HBV or exposed to AFB1 alone. These results provided further support for the existence of the synergistic effect between HBV and AFB1 in tree shrew’s hepatocarcinogenesis[13-15]. Even though only one case of HCC developed in group B, which was infected with HBV alone, proliferation foci and/or nodules appeared quite early and frequently in this group. One tree shrew in group B which died at the 120th wk was found with 2 proliferating nodules with 0.5 cm in diameter in the liver. These results indicate that HBV has the capability of inducing liver cancer, but its capability is weak.
Wild type p53 gene is the control gene at G1 phase of cell cycle. It can inhibit DNA-damaged cell from entering G1 phase and let the cell repair the damage[16]. Mutated p53 gene not only loses the functions that wild type p53 has but also promotes malignant transformation of cells[17]. All the liver tissues of tree shrews in all groups were negative for mutant p53 protein by immunohistochemistry in early period of hepatocarcinogenesis. However, mutant p53 proteins were detectable in the middle stage of hepatocarcinogenesis, before the appearance of HCC. This demonstrates that p53 mutation occurs prior to the appearance of HCC. In the control group, no HCC developed and no mutant p53 proteins and mutations of p53 gene were detected, suggesting that the p53 mutation is a crucial factor to initiate the malignant transformation of cell. The results of examining the status of p53 gene by Southern blotting showed that 2 liver samples biopsied at the 45th wk in group C had an abnormal band of 3.8 kb. This indicates that the alteration of p53 gene in the hepatocyte of tree shrew is dependent on a cumulative amount of AFB1 and sufficient time, and also supports the finding that mutation of p53 occurs before the appearance of HCC. The reason for loss expression of mutant p53 in those 2 animals may be due to the low level of mutant p53 protein. In the 8 samples sent to South Korea, p53 mutation was not observed in 3 normal liver tissues, but 3 poorly-differentiated cases from the 5 HCC samples showed p53 mutations. The rate of mutation was consistent with our previous report[18]. It was also similar to the reports on human samples. The 3 tree shrews containing p53 mutation gene were from group A treated by both HBV and AFB1, implying that HBV and AFB1 may play a synergistic role in p53 mutation. This may be one of mechanisms that HBV synergies AFB1 in hepatocarcinogenesis. p53 mutations were located at codons 275, 78 and 13, respectively. No mutation was found at codon 249. It was differently from the mutation at codon 249 of the p53 gene identified as a hotspot mutation in hepatocellular carcinomas occurring in populations exposed to AFB1 and HBV[4,5,19,20]. However, it was similar to the results of studies on non-human primate animal models[21]. This discrepancy may be due to the different species, or too small amount of cases that were detected to find any mutation of p53 at codon 249. Moreover, it also suggests that p53 mutation, which is closely related to the development of HCC, does not merely occur at codon 249.
In HCC ras was first proved as one of the transforming genes, which belong to G-protein family gene. When it is converted to active oncogene by point mutation or gene amplification the signal transmission of cell membranes may change, which drives cell division, and results in abnormal differentiation and finally neoplasm formation. Oncogene ras directly takes part in human carcinogenesis, perhaps accounting for as many as 15%-20% of all human tumors. It is well documented that the ras gene product, p21 protein, has GTPase activity and is involved in signal transduction. p21 is now well recognized for its essential function in transducing extracellular signals that regulate cell growth, survival, and differentiation. Overexpression and point mutations of ras gene were not only found in HCC[22-23] but also found in liver cirrhosis and the correlation with liver cell dysplasia[24]. We detected the p21 expression by immunohi-stochemistry in biopsied liver tissues of tree shrews at the 45th, 105th and 119th wk. The results showed that the accumulative total of positive rate for p21 in group A was 41.02%, which was significantly higher than that in group B (7.14%) and group C (24.13%). This indicates that HBV and AFB1 can synergistically activate ras gene in hepatocyte resulting in overexpression of p21[25]. The p21 protein overexpression appeared before HCC development, indicating that the p21 protein overexpression is the early event during hepatocarcinogenesis and p21 protein may be an early marker in the development of HCC[26]. In the present study, among the 25 animals that were positive for p21, 20 (80%) developed HCC whereas only 10 of 91 (10.99%) p21-negative animals developed HCC at the end of the experiment. This suggests that overexpression of p21 plays an important role in the development of HCC.
Comparison of the nucleotide and amino acid, sequences of human wild-type p53 the structural homology was higher between tree shrews and human than between tree shrews and mouse[11], indicating the tree shrew model is a useful animal model to study the etiology and pathogenesis of HCC in humans[27].
Edited by Zhu LH and Chen WW Proofread by Xu FM
| 1. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 2. | Wang JS, Huang T, Su J, Liang F, Wei Z, Liang Y, Luo H, Kuang SY, Qian GS, Sun G. Hepatocellular carcinoma and aflatoxin exposure in Zhuqing Village, Fusui County, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2001;10:143-146. [PubMed] |
| 3. | Smela ME, Hamm ML, Henderson PT, Harris CM, Harris TM, Essigmann JM. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc Natl Acad Sci USA. 2002;99:6655-6660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Jackson PE, Qian GS, Friesen MD, Zhu YR, Lu P, Wang JB, Wu Y, Kensler TW, Vogelstein B, Groopman JD. Specific p53 mutations detected in plasma and tumors of hepatocellular carcinoma patients by electrospray ionization mass spectrometry. Cancer Res. 2001;61:33-35. [PubMed] |
| 5. | Shimizu Y, Zhu JJ, Han F, Ishikawa T, Oda H. Different frequencies of p53 codon-249 hot-spot mutations in hepatocellular carcinomas in Jiang-su province of China. Int J Cancer. 1999;82:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hy-droxy-2-nonenal, preferentially forms DNA adducts at condon 249 of human p53 gene, a unique mutational hotspot in hepa-tocellular carcinoma. Carcinogenesis. 2002;23:1781-1789. [RCA] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 872] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 8. | Wei S, Kito K, Miyoshi A, Matsumoto S, Kauzi A, Aramoto T, Abe Y, Ueda N. Incidence of p53 and ras gene mutations in DMBA-induced rat leukemias. J Exp Clin Cancer Res. 2002;21:389-396. [PubMed] |
| 9. | Basolo F, Pinchera A, Fugazzola L, Fontanini G, Elisei R, Romei C, Pacini F. Expression of p21 ras protein as a prognostic factor in papillary thyroid cancer. Eur J Cancer. 1994;30A:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Diaz R, Lopez-Barcons L, Ahn D, Garcia-Espana A, Yoon A, Matthews J, Mangues R, Perez-Soler R, Pellicer A. Complex effects of Ras proto-oncogenes in tumorigenesis. Carcinogenesis. 2004;25:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Park US, Su JJ, Ban KC, Qin L, Lee EH, Lee YI. Mutations in the p53 tumor suppressor gene in tree shrew hepatocellular carcinoma associated with hepatitis B virus infection and intake of aflatoxin B1. Gene. 2000;251:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Yan RQ, Su JJ, Huang DR, Gan YC, Yang C, Huang GH. Human hepatitis B virus and hepatocellular carcinoma. II. Experimental induction of hepatocellular carcinoma in tree shrews exposed to hepatitis B virus and aflatoxin B1. J Cancer Res Clin Oncol. 1996;122:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Bannasch P, Khoshkhou NI, Hacker HJ, Radaeva S, Mrozek M, Zillmann U, Kopp-Schneider A, Haberkorn U, Elgas M, Tolle T. Synergistic hepatocarcinogenic effect of hepadnaviral infection and dietary aflatoxin B1 in woodchucks. Cancer Res. 1995;55:3318-3330. [PubMed] |
| 14. | Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Li Y, Su JJ, Qin LL, Yang C, Ban KC, Yan RQ. Synergistic effect of hepatitis B virus and aflatoxin B1 in hepatocarcinogenesis in tree shrews. Ann Acad Med Singapore. 1999;28:67-71. [PubMed] |
| 16. | Offer H, Erez N, Zurer I, Tang X, Milyavsky M, Goldfinger N, Rotter V. The onset of p53-dependent DNA repair or apoptosis is determined by the level of accumulated damaged DNA. Carcinogenesis. 2002;23:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Bálint E E, Vousden KH. Activation and activities of the p53 tumour suppressor protein. Br J Cancer. 2001;85:1813-1823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Su JJ, Qin GZ, Yan RQ, Huang DR, Yang C, Huang GH, Lotlikar PD. Expression of p53 gene in hepatocellular carcinomas in-duced by aflatoxin B1 with or without human hepatitis B virus in tree shrews. Exp Mol Med. 1997;29:177-182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Zhu M, Dai Y, Zhan R. [HBxAg enhanced p53 protein accumulation in hepatoma cells]. Zhonghua Binglixue Zazhi. 1999;28:31-34. [PubMed] |
| 20. | Lunn RM, Zhang YJ, Wang LY, Chen CJ, Lee PH, Lee CS, Tsai WY, Santella RM. p53 mutations, chronic hepatitis B virus infection, and aflatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res. 1997;57:3471-3477. [PubMed] |
| 21. | Rivkina MB, Cullen JM, Robinson WS, Marion PL. State of the p53 gene in hepatocellular carcinomas of ground squirrels and woodchucks with past and ongoing infection with hepadnaviruses. Cancer Res. 1994;54:5430-5437. [PubMed] |
| 22. | Weihrauch M, Benicke M, Lehnert G, Wittekind C, Wrbitzky R, Tannapfel A. Frequent k- ras -2 mutations and p16(INK4A)methylation in hepatocellular carcinomas in workers exposed to vinyl chloride. Br J Cancer. 2001;84:982-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Parsons BL, Culp SJ, Manjanatha MG, Heflich RH. Occurrence of H-ras codon 61 CAA to AAA mutation during mouse liver tumor progression. Carcinogenesis. 2002;23:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Feng Z, He R, Lu Z, Ling Y. [Expression of ras oncogene p21 product and proliferating cell nuclear antigen in liver cirrhosis and the correlation with liver cell dysplasia]. Zhonghua Ganzangbing Zazhi. 2000;8:343-345. [PubMed] |
| 25. | Su JJ, Qin GZ, Yan RQ, Huang DR, Yang C, Lotlikar PD. The expression of insulin-like growth factor II, hepatitis B virus X antigen and p21 in experimental hepatocarcinogenesis in tree shrews. Ann Acad Med Singapore. 1999;28:62-66. [PubMed] |
| 26. | Fearon ER. K-ras gene mutation as a pathogenetic and diagnostic marker in human cancer. J Natl Cancer Inst. 1993;85:1978-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Cao J, Yang EB, Su JJ, Li Y, Chow P. The tree shrews: adjuncts and alternatives to primates as models for biomedical research. J Med Primatol. 2003;32:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |