Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3490
Revised: March 22, 2004
Accepted: April 7, 2004
Published online: December 1, 2004
AIM: To investigate the inhibitory effect of heparin-derived oligosaccharides (Oligs) on secretion of interleukin-4 (IL-4) and interleukin-5 (IL-5) from human peripheral blood T lymphocytes (PBTLs).
METHODS: Oligs were prepared by three different heparin depolymerization methods and separated by gel filtration chromatography. PBTLs from ten adult patients with allergic eosinophilic gastroenteritis were treated with phytahematoagglutinin (PHA) and Oligs. The supernatants from the cell culture of PBTLs were harvested and subjected to the determination of IL-4 and IL-5 contents by ELISA method.
RESULTS: At the concentration of 5 μg/mL, Oligs with different Mr had different effects on the secretion of IL-4 and IL-5. The tetrasaccharide with Mr of 1142, produced by depolymerizing heparin with hydrogen peroxide, had the strongest inhibitory effect on the secretion of IL-4. It decreased the IL-4 content from 375.6 ± 39.2 ng/L (PHA group) to 12.5 ± 5.7 ng/L (P < 0.01). The hexasaccharide with Mr of 1806, produced by depolymerizing heparin with β -elimination method, had the strongest inhibitory effect on the secretion of IL-5. It decreased the IL-5 content from 289.2 ± 33.4 ng/L (PHA group) to 22.0 ± 5.2 ng/L (P < 0.01).
CONCLUSION: The inhibitory activity of Oligs on the secretion of IL-4 and IL-5 from human PBTLs closely depends on their molecular structure, and there may be an essential structure to act as an inhibitor. The most effective inhibitors of IL-4 and IL-5 secretion are tetrasaccharides and hexasaccharides, respectively.
- Citation: Ji SL, Cui HF, Shi F, Chi YQ, Cao JC, Geng MY, Guan HS. Inhibitory effect of heparin-derived oligosaccharides on secretion of interleukin-4 and interleukin-5 from human peripheral blood T lymphocytes. World J Gastroenterol 2004; 10(23): 3490-3494
- URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3490.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3490
Heparin is a highly sulfated, polyanionic glycosaminoglycan. It is composed of the trisulfated disaccharide unit [- L-iduronic acid-2-sulfate (IdoA2S) (1→4)-α -D- glucosamine-N,6 -disulfate (GlcNS6S)-], interrupted by irregular sequences containing undersulfated (or oversulfated) uronic acids and amino sugar residues, with an average relative molecular mass (Mr) of 12000-15000[1]. In addition to its anticoagulatory effect, heparin has anti-inflammatory property, which has been used for treatment of ulcerative colitis and other inflammatory bowel diseases (IBD)[2-15]. Although many studies have reported heparin’s anti-inflammatory activities, there is little information available in literature about the specific oligosaccharide structures in heparin that inhibits the secretion of IL-4 and IL-5. IL-4 and IL-5 abnormalities have been reported to be consistent with the elevated IgE and eosinophilia in allergic eosinophilic gastroenteritis, suggesting that strategies targeting T lymphocytes may be efficacious in treatment of this kind of diseases[16-19]. Here, we examined whether there was a relationship between the molecular weight of heparin-derived Oligs and their inhibitory effect on IL-4 and IL-5 secretion from PBTLs in allergic eosinophilic gastroenteritis.
Heparin sodium was donated by Dongying Tiandong Biochemical Industrial Co. Ltd, and 300 mL/L hydrogen peroxide (H2O2) was purchased from Laiyang Fine Chemical Regent Co, Ltd. Benzethonium chloride and (4-chloro)-benzyl chloride were purchased from Sigma Co., USA. Sodium nitrite was purchased from Jinan Chemical Reagents Co., Ltd. Superdex-30 was purchased from Amersham Biosciences. Human IL-4 and IL-5 ELISA kits (96 tests) were purchased from Bender Medsystems, USA. Phytahematoagglutinin (PHA) was purchased from Shanghai Yihua Medical Science and Technology Co., Ltd. RPMI 1640 culture medium was purchased from Gibco, Paisley, UK.
Heparin was degraded by oxidative depolymerization with hydrogen peroxide[20], deaminative cleavages with nitrous acid[21] and β -eliminative cleavages with alkaline[22], respectively. All Oligs were obtained from the products separated by gel filtration chromatography with Superdex-30 column (26 mm × 1200 mm), and the oligosaccharides were eluted with 0.25 mol/L ammonium bicarbonate and dried with lyophilization.
The Mr of Oligs was determined by high performance size exclusion chromatography (HPSEC) on two Ultrahydrogel 250 (7.8 mm × 300 mm) columns in series. The calibration was based on low molecular mass heparin for calibration CRS (Batch No 1A, European Pharmacopoeia) standards with number average molecular mass (Mn) of 3700. A refractometer (RI) detector connected in series to an ultraviolet (UV) spectrophotometer set at 234 nm was used for detection, and 28.4 g/L solution of sodium sulphate (pH 5.0) was used as the mobile phase at a flow rate of 0.5 mL/min[23].
Twenty-five milliliters of edetic acid anticoagulant peripheral blood was taken from ten adult patients with allergic eosinophilic gastroenteritis, and then mixed with 25 mL of PBS ( pH 7.4, containing 50 mL/L fetal bovine serum). The diluted peripheral blood was then laid on 50 mL of lymphocyte separating solution carefully and centrifuged for 20 min at 2000 r/min at room temperature. The peripheral blood lymphocytes (PBL) were collected and washed two times with PBS and suspended in RPMI 1640 solution (containing 200 mL/L fetal bovine serum). One millilitre of PBL suspending solution was added to the column of Nylon cotton (5 mm×16 mm). Then 0.2 mL of RPMI 1640 (containing 200 mL/L fetal bovine serum) was added on the Nylon cotton surface to close the column. The column was incubated for 30 min at 37 °C in an incubator. After that, PBTLs were eluted with RPMI 1640 (containing 200 mL/L fetal bovine serum) solution and then centrifuged for 10 min at 2000 r/min. PBTLs were diluted to the cell density of 1.0 × 10 6 cells/mL, and 0.2 mL of the diluted cell suspending solution was added to a well of 96-well plate as a blank control. Another well was added into 0.2 mL of diluted cell suspending solution containing 20 µg of PHA as PHA control group. Other wells were added into 0.2 mL of diluted cell suspending solution containing 20 µg of PHA and 1 µL of 1.0 mg/mL heparin and Oligs. The cells were incubated for 48 h at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air.
The cells were harvested and centrifuged for 10 min at 1000 r/min at room temperature. IL-4 and IL-5 in the cell culture solution were assayed by ELISA according to the manufacturer’s instructions.
Data were expressed as mean ± SD. Student’s t test and one-way analysis of variance were used for statistical analysis. P values less than 0.05 were considered statistically significant.
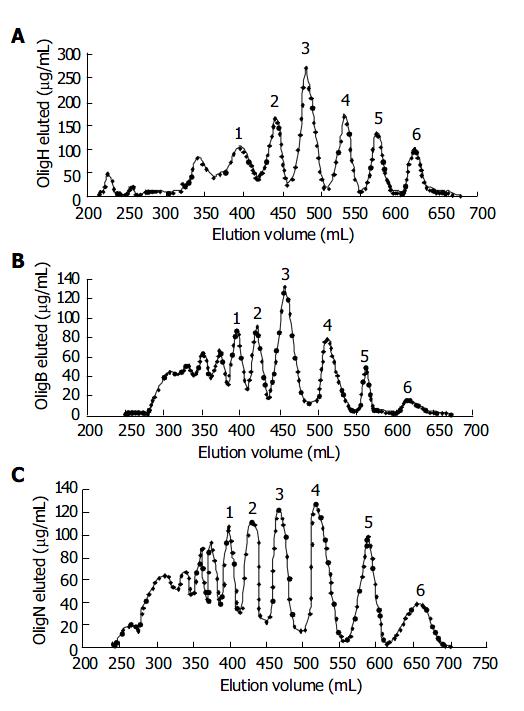
Figure 1 shows the Oligs prepared from heparin degradation and separated by gel filtration chromatography. The peaks numbered were Oligs collected for inhibiting IL-4 and IL-5 experiments, and their Mr is listed in Table 1.
| Oligs produced by H2O2 oxidation | Oligs produced byβ -elimination | Oligs produced by degradation with HNO2 | |||
| Samples | Mr | Samples | Mr | Samples | Mr |
| Olig H1 | 6032 | Olig B1 | 6621 | Olig N1 | 6680 |
| Olig H2 | 3206 | Olig B2 | 3375 | Olig N2 | 3150 |
| Olig H3 | 2381 | Olig B3 | 2447 | Olig N3 | 2334 |
| Olig H4 | 1786 | Olig B4 | 1804 | Olig N4 | 1747 |
| Olig H5 | 1142 | Olig B5 | 1344 | Olig N5 | 1047 |
| Olig H6 | 632 | Olig B6 | 702 | Olig N6 | 609 |
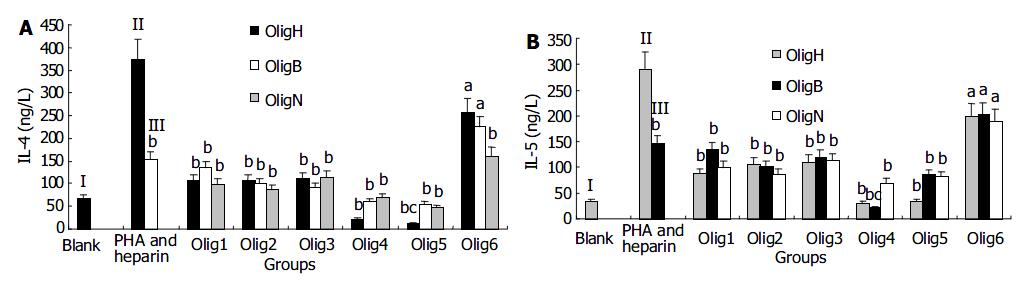
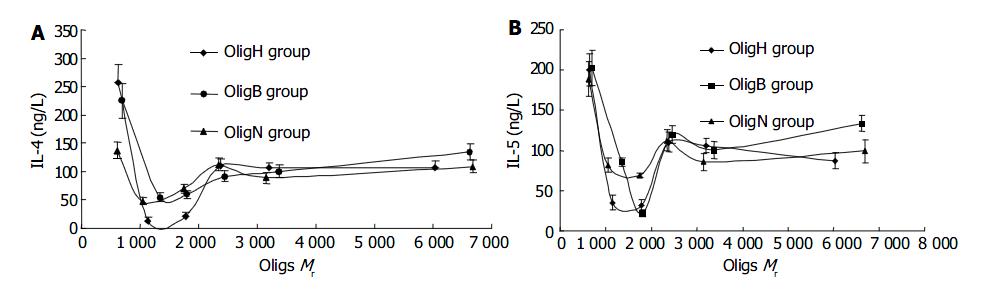
The effect of Oligs on secretion of IL-4 showed that all the Oligs had inhibitory activities and Oligs prepared from different methods or with different Mr had different effects. First, the Olig prepared from H2O2 depolymerizing method, was Olig H5 with Mr of 1142 (tetrasaccharides), which had the strongest inhibitory effect and decreased the IL-4 content from 375.6 ± 39.2 ng/L (PHA group) to 12.5 ± 5.7 ng/L ( P < 0.01). Second, among the Oligs from β -eliminative cleavage heparin, Olig B5 with Mr of 1 344 (tetrasaccharides) had the strongest inhibitory activity and decreased the IL-4 content to 54.4 ± 6.3 ng/L ( P < 0.01). Third, the Olig from nitrous acid deaminative cleavage heparin, was Olig N5 with Mr of 1107 (tetrasaccharides), which had the strongest inhibitory activity and decreased the IL-4 content to 47.4 ± 5.8 ng/L (P < 0.01). Although all these Oligs were tetrasaccharides, they had different Mr, because the amount of sulfate groups in their structure was different. The IL-4 content in the heparin group was 152.4 ± 17.9 ng/L, and the difference in inhibitory activities between Oligs and heparin group was significant (P < 0.01, Figure 2A, Figure 3A).
The inhibitory effect of Oligs on secretion of IL-5 and IL-4 was similar. But there were some differences. Firstly, the Oligs prepared from H2O2 oxidation method, were Olig H4 with Mr of 1 786 and Olig H5 with Mr of 1 142, which had strongest inhibitory activities. They decreased the IL-5 content from 289.2 ± 33.4 ng/L (PHA group) to 31.7 ± 5.6 ng/L and 35.5 ± 4.4 ng/L, respectively (P < 0.01). Secondly, among the Oligs from β -eliminative cleavage heparin, Olig B4 with Mr of 1 804 (hexasaccharides) had the strongest inhibitory activity and decreased the IL-5 content to 22.0 ± 5.2 ng/L ( P < 0.01). Thirdly, among the Oligs from nitrous acid deaminative cleavage heparin, Olig N4 with Mr of 1747 (hexasaccharides) had the strongest inhibitory activity and decreased the IL-5 content to 69.2 ± 6.3 ng/L ( P < 0.01). The inhibitory activities of these Oligs were stronger than those of unfractioned heparin (P < 0.01, Figure 2B). The strongest inhibitor was Olig B4 (Figure 3B).
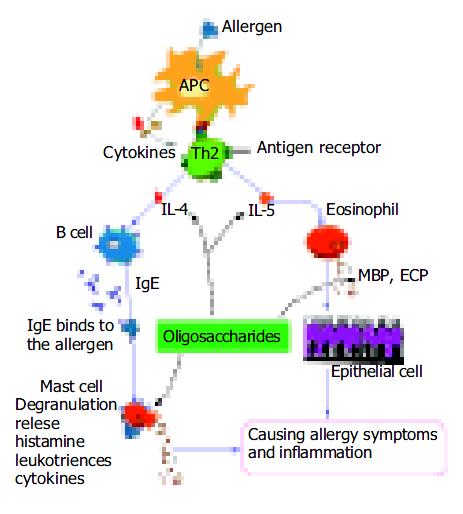
Allergic eosinophilic gastroenteritis is characterized by elevated total immunoglobulin E (IgE). IgE plays a significant role in allergic IBD by mediating the cross-linking of high-affinity Fc receptors (FcЄ RI) on mast cells, thus resulting in the release of a vast array of pro-inflammatory mediators including histamine, leukotrienes, and cytokines[24] (Figure 4). In clinical studies, increased secretion of IL-4 and IL-5 by peripheral blood T cells has been reported in patients with eosinophilic gastroenteritis[17]. Furthermore, T cells derived from the duodenum of patients with eosinophilic gastrointestinal disorder could preferentially secrete helper T cell 2 (Th2) cytokines that pre-dominantly include IL-4 and IL-5 when stimulated with milk proteins[17]. IL-4 might play a critical role in mediating IgE-dependent allergic reactions, and regulation of IL-4 production or action might be useful for the prevention or therapy of immediate hypersensitivity disorders[25-27]. IL-5, on the other hand, is most specific to the eosinophil lineage and responsible for the selective expansion of eosinophils and their release from bone marrow. Eosinophil granules contain a crystalloid core composed of major basic proteins (MBP) and a matrix composed of eosinophil cationic protein (ECP)[28] (Figure 4). These cationic proteins share certain pro-inflammatory properties but differ in other aspects. MBP also triggers degranulation of mast cells and basophils. Triggering of eosinophils through engagement of receptors for cytokines could lead to the generation of a wide range of inflammatory cytokines, including IL-1, IL-3, IL-4, IL-5, IL-13, granulocyte-macrophage colony stimulating factor (GM-CSF), transforming growth factors (TGF), TNF-α , RANTES, macrophage inflammatory protein 1α , vascular endothelial cell growth factor, and eotaxin 1, indicating that they have the potential to modulate multiple aspects of the immune response[29]. These findings imply that regulation of IL-5 production or action may also be useful for the prevention or therapy of allergy symptoms and inflammation.
Heparin is a kind of polyanionic polysaccharides. In addition to its anticoagulant activity, heparin has a wide range of biological activities, including inhibition of complement activation[30], regulation of cell proliferation[31], inhibition of angiogenesis and tumor growth[32,33], and antiviral activity[34,35]. Over the last decade, heparin and low molecular mass heparin have been used to treat IBD in clinical practice[36-50]. The mechanisms by which heparin is able to treat IBD include its ability to inhibit the recruitment of neutrophils, reduce production of pro-inflammatory cytokines[51] and restore the high-affinity receptor binding to antiulcerogenic growth factor[12,13]. The ability of heparin to inhibit neutrophil activation, adhesion, and chemotaxis was also found in a mouse model of IBD[14], suggesting that balanced interactions between mast cells and neutrophils might be important for the development of IBD. Furthermore, unfractioned heparin has potent immunomodulatory effects[52-57]. Administration of unfractioned heparin may therefore be rational in patients with ulcerative colitis or Crohn’s disease resistant to conventional forms of treatment[39,40]. The anti-inflammatory effects of heparin can be most probably attributed to its physical binding to a variety of heparin-binding proteins such as TNF-α , IL-4, IL-5, RANTES, secretory leukocyte protease inhibitor, neutrophil-derived elastase and cathepsin G, eosinophil-derived major basic protein, and L- and P-selectins[58-61]. Alternatively, heparin has been shown to specifically inhibit the inositol 1,4, 5-triphosphate signal transduction pathway, which is important for a vast array of inflammatory cellular responses[62,63].
If heparin is to be used as an anti-inflammatory drug, the risk of inducing bleeding must be abrogated. It has been reported that the anti-inflammatory effects of heparin were independent of its anticoagulant activity[64-67], especially when it was used for treatment of allergic inflammation. Partial chemical modifications of heparin, such as depolymerization or partial desulfation, are the aim to develop heparin-derived anti-inflammatory drugs. We degraded heparin and separated the fragments by gel filtration chromatography. The oligosaccharides obtained were used as anti-inflammatory reagents and the results were promising. The hypothetic mechanisms for anti-inflammatory effects, such as inhibiting secretion of IL-4 and IL-5, were studied. Our results showed that Oligs with different Mr had different activities on inhibiting the secretion of IL-4 and IL-5. The Oligs, which had the strongest inhibitory activities on IL-4 secretion, were tetrasaccharides, but they had different Mr corresponding to the production methods. That was because different methods caused different desulfation during the process of degradation. The same phenomenon occured in inhibiting the secretion of IL-5, but the strongest inhibitors were hexasaccharides other than tetrasaccharides. The mechanism needs further studies.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Casu B. Heparin structure. Haemostasis. 1990;20 Suppl 1:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Gaffney PR, Doyle CT, Gaffney A, Hogan J, Hayes DP, Annis P. Paradoxical response to heparin in 10 patients with ulcerative colitis. Am J Gastroenterol. 1995;90:220-223. [PubMed] |
| 3. | Evans RC, Wong VS, Morris AI, Rhodes JM. Treatment of corticosteroid-resistant ulcerative colitis with heparin--a report of 16 cases. Aliment Pharmacol Ther. 1997;11:1037-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Yoshikane H, Sakakibara A, Ayakawa T, Taki N, Kawashima H, Arakawa D, Hidano H. Disseminated intravascular coagulation in an ulcerative colitis case not associated with surgery. Hepatogastroenterology. 2000;47:1608-1610. [PubMed] |
| 5. | Cui HF, Jiang XL. Treatment of corticosteroid-resistant ulcerative colitis with oral low molecular weight heparin. World J Gastroenterol. 1999;5:448-450. [PubMed] |
| 6. | Ang YS, Mahmud N, White B, Byrne M, Kelly A, Lawler M, McDonald GS, Smith OP, Keeling PW. Randomized comparison of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Folwaczny C, Wiebecke B, Loeschke K. Unfractioned heparin in the therapy of patients with highly active inflammatory bowel disease. Am J Gastroenterol. 1999;94:1551-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Dotan I, Hallak A, Arber N, Santo M, Alexandrowitz A, Knaani Y, Hershkoviz R, Brazowski E, Halpern Z. Low-dose low-molecular weight heparin (enoxaparin) is effective as adjuvant treatment in active ulcerative colitis: an open trial. Dig Dis Sci. 2001;46:2239-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Vrij AA, Jansen JM, Schoon EJ, de Bruïne A, Hemker HC, Stockbrügger RW. Low molecular weight heparin treatment in steroid refractory ulcerative colitis: clinical outcome and influence on mucosal capillary thrombi. Scand J Gastroenterol Suppl. 2001;234:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Törkvist L, Thorlacius H, Sjöqvist U, Bohman L, Lapidus A, Flood L, Agren B, Raud J, Löfberg R. Low molecular weight heparin as adjuvant therapy in active ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1323-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Papa A, Danese S, Gasbarrini A, Gasbarrini G. Review article: potential therapeutic applications and mechanisms of action of heparin in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Michell NP, Lalor P, Langman MJ. Heparin therapy for ulcerative colitis? Effects and mechanisms. Eur J Gastroenterol Hepatol. 2001;13:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Day R, Forbes A. Heparin, cell adhesion, and pathogenesis of inflammatory bowel disease. Lancet. 1999;354:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Wan MX, Liu Q, Wang Y, Thorlacius H. Protective effect of low molecular weight heparin on experimental colitis: role of neutrophil recruitment and TNF-alpha production. Inflamm Res. 2002;51:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Fries W, Pagiaro E, Canova E, Carraro P, Gasparini G, Pomerri F, Martin A, Carlotto C, Mazzon E, Sturniolo GC. The effect of heparin on trinitrobenzene sulphonic acid-induced colitis in the rat. Aliment Pharmacol Ther. 1998;12:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Lalani T, Simmons RK, Ahmed AR. Biology of IL-5 in health and disease. Ann Allergy Asthma Immunol. 1999;82:317-32; quiz 332-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Jaffe JS, James SP, Mullins GE, Braun-Elwert L, Lubensky I, Metcalfe DD. Evidence for an abnormal profile of interleukin-4 (IL-4), IL-5, and gamma-interferon (gamma-IFN) in peripheral blood T cells from patients with allergic eosinophilic gastroenteritis. J Clin Immunol. 1994;14:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. 1999;29:1496-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417-4422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Fussi F. Process for obtaining low molecular weight heparins endowed with elevated pharmacological properties, and prod-uct so obtained. United States Patent. 1981;4:108-281. |
| 21. | Lormeau JC, Petitou M, Choay J, Choay SA. Oligosaccharides having anti-Xa activity and pharmaceutical compositions con-taining them. United States Patent. 1998;35:770. |
| 22. | Uzan A, Rhone-Poulenc Rorer SA. Sulfated polysaccharides obtained from heparin, preparation process, pharmaceutical composition and use thereof. United States Patent. 1998;5:721-849. |
| 23. | Kristensen HI, Tromborg EM, Nielsen JR, Nielsen JI, Johansen KB, Ostergaard PB. Development and validation of a size exclusion chromatography method for determination of molecular masses and molecular mass distribution in low molecular weight heparin. Thromb Res. 1991;64:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, Costa JJ, Galli SJ. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J Immunol. 1999;162:5455-5465. [PubMed] |
| 25. | Madden KB, Urban JF, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387-1391. [PubMed] |
| 26. | Schreiber S, Heinig T, Panzer U, Reinking R, Bouchard A, Stahl PD, Raedler A. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterology. 1995;108:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | West GA, Matsuura T, Levine AD, Klein JS, Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 553] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 29. | Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis (Review). Int J Mol Med. 1998;1:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Sharath MD, Merchant ZM, Kim YS, Rice KG, Linhardt RJ, Weiler JM. Small heparin fragments regulate the amplification pathway of complement. Immunopharmacology. 1985;9:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Wright TC, Castellot JJ, Petitou M, Lormeau JC, Choay J, Karnovsky MJ. Structural determinants of heparin's growth inhibitory activity. Interdependence of oligosaccharide size and charge. J Biol Chem. 1989;264:1534-1542. [PubMed] |
| 32. | Folkman J, Langer R, Linhardt RJ, Haudenschild C, Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 437] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985;230:1375-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 328] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Holodniy M, Kim S, Katzenstein D, Konrad M, Groves E, Merigan TC. Inhibition of human immunodeficiency virus gene amplification by heparin. J Clin Microbiol. 1991;29:676-679. [PubMed] |
| 35. | Shieh MT, Spear PG. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J Virol. 1994;68:1224-1228. [PubMed] |
| 36. | Head KA, Jurenka JS. Inflammatory bowel disease Part 1: ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8:247-283. [PubMed] |
| 37. | Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, Janbon C, Leizorovicz A, Olsson CG, Turpie AG. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Fibrinolysis. 2003;14:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Jani N, Regueiro MD. Medical therapy for ulcerative colitis. Gastroenterol Clin North Am. 2002;31:147-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Prajapati DN, Newcomer JR, Emmons J, Abu-Hajir M, Binion DG. Successful treatment of an acute flare of steroid-resistant Crohn's colitis during pregnancy with unfractionated heparin. Inflamm Bowel Dis. 2002;8:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Papa A, Danese S, Gasbarrini A, Gasbarrini G. Review article: potential therapeutic applications and mechanisms of action of heparin in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Kassis J, Fugère F, Dubé S. The safe use of epidural anesthesia after subcutaneous injection of low-dose heparin in general abdominal surgery. Can J Surg. 2000;43:289-294. [PubMed] |
| 42. | Ang YS, Mahmud N, White B, Byrne M, Kelly A, Lawler M, McDonald GS, Smith OP, Keeling PW. Randomized comparison of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Katz S. Update in medical therapy in inflammatory bowel disease: a clinician's view. Dig Dis. 1999;17:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Koutroubakis IE, Sfiridaki A, Mouzas IA, Maladaki A, Kapsoritakis A, Roussomoustakaki M, Kouroumalis EA, Manousos ON. Resistance to activated protein C and low levels of free protein S in Greek patients with inflammatory bowel disease. Am J Gastroenterol. 2000;95:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Day R, Ilyas M, Daszak P, Talbot I, Forbes A. Expression of syndecan-1 in inflammatory bowel disease and a possible mechanism of heparin therapy. Dig Dis Sci. 1999;44:2508-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Haslam N, Standen GR, Probert CS. An investigation of the association of the factor V Leiden mutation and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:1289-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Törkvist L, Thorlacius H, Sjöqvist U, Bohman L, Lapidus A, Flood L, Agren B, Raud J, Löfberg R. Low molecular weight heparin as adjuvant therapy in active ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1323-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Day R, Forbes A. Heparin, cell adhesion, and pathogenesis of inflammatory bowel disease. Lancet. 1999;354:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | McCarty MF. Vascular heparan sulfates may limit the ability of leukocytes to penetrate the endothelial barrier--implications for use of glucosamine in inflammatory disorders. Med Hypotheses. 1998;51:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Korzenik JR, Hsu A, Robert ME. Effect of heparin on dextran sulfate sodium-induced colitis. Dig Dis Sci. 1998;43:1800-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Papa A, Danese S, Gasbarrini A, Gasbarrini G. Review article: potential therapeutic applications and mechanisms of action of heparin in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Ahmed T, Abraham WM, D'Brot J. Effects of inhaled heparin on immunologic and nonimmunologic bronchoconstrictor responses in sheep. Am Rev Respir Dis. 1992;145:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Bowler SD, Smith SM, Lavercombe PS. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am Rev Respir Dis. 1993;147:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Chang NS, Intrieri C, Mattison J, Armand G. Synthetic polysulfated hyaluronic acid is a potent inhibitor for tumor necrosis factor production. J Leukoc Biol. 1994;55:778-784. [PubMed] |
| 55. | Lantz M, Thysell H, Nilsson E, Olsson I. On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest. 1991;88:2026-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253-3258. [PubMed] |
| 57. | Teixeira MM, Hellewell PG. Suppression by intradermal administration of heparin of eosinophil accumulation but not oedema formation in inflammatory reactions in guinea-pig skin. Br J Pharmacol. 1993;110:1496-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Lider O, Mekori YA, Miller T, Bar-Tana R, Vlodavsky I, Baharav E, Cohen IR, Naparstek Y. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. Eur J Immunol. 1990;20:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Fath MA, Wu X, Hileman RE, Linhardt RJ, Kashem MA, Nelson RM, Wright CD, Abraham WM. Interaction of secretory leukocyte protease inhibitor with heparin inhibits proteases involved in asthma. J Biol Chem. 1998;273:13563-13569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Tyrell DJ, Kilfeather S, Page CP. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci. 1995;16:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Jones CA, Williams KA, Finlay-Jones JJ, Hart PH. Interleukin 4 production by human amnion epithelial cells and regulation of its activity by glycosaminoglycan binding. Biol Reprod. 1995;52:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Ghosh TK, Eis PS, Mullaney JM, Ebert CL, Gill DL. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988;263:11075-11079. [PubMed] |
| 63. | Ahmed T, Syriste T, Mendelssohn R, Sorace D, Mansour E, Lansing M, Abraham WM, Robinson MJ. Heparin prevents antigen-induced airway hyperresponsiveness: interference with IP3-mediated mast cell degranulation? J Appl Physiol (1985). 1994;76:893-901. [PubMed] |
| 64. | Sy MS, Schneeberger E, McCluskey R, Greene MI, Rosenberg RD, Benacerraf B. Inhibition of delayed-type hypersensitivity by heparin depleted of anticoagulant activity. Cell Immunol. 1983;82:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Xie X, Rivier AS, Zakrzewicz A, Bernimoulin M, Zeng XL, Wessel HP, Schapira M, Spertini O. Inhibition of selectin-mediated cell adhesion and prevention of acute inflammation by nonanticoagulant sulfated saccharides. Studies with carboxyl-reduced and sulfated heparin and with trestatin a sulfate. J Biol Chem. 2000;275:34818-34825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Fryer A, Huang YC, Rao G, Jacoby D, Mancilla E, Whorton R, Piantadosi CA, Kennedy T, Hoidal J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J Pharmacol Exp Ther. 1997;282:208-219. [PubMed] |
| 67. | Kariya Y, Kyogashima M, Suzuki K, Isomura T, Sakamoto T, Horie K, Ishihara M, Takano R, Kamei K, Hara S. Preparation of completely 6-O-desulfated heparin and its ability to enhance activity of basic fibroblast growth factor. J Biol Chem. 2000;275:25949-25958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |