Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3459
Revised: February 20, 2004
Accepted: February 24, 2004
Published online: December 1, 2004
AIM: To investigate the anticancer activity of Honokiol on RKO, a human colorectal carcinoma cell line in vitro and in vivo, and to evaluate its possible use in clinic.
METHODS: in vitro anticancer activity of honokiol was demonstrated by its induction of apoptosis in tumor cells. We analyzed cell proliferation with MTT assay, cell cycle with flow cytosmeter, DNA fragment with electrophoresis on agarose gels. To test the mechanism of honokiol-induced apoptosis, Western blotting was used to investigate the factors involved in this process. The pharmacokinetics study of honokiol was tested by high phase liquid chromatography. In in vivo study, Balb/c nude mice were incubated with RKO cells. Honokiol was injected intraperitoneally every other day into tumor bearing Balb/c nude mice.
RESULTS: Our results showed that honokiol induced apoptosis of RKO cells in a time- and dose-dependent manner. At 5-10 ug/mL for 48 h, honokiol induced apoptosis through activating Caspase cascades. Pharmacokinetics study demonstrated that, honokiol could be absorbed quickly by intraperitoneal injection, and maintained in plasma for more than 10 h. In nude mice bearing RKO-incubated tumor, honokiol displayed anticancer activity by inhibiting tumor growth and prolonging the lifespan of tumor bearing mice.
CONCLUSION: With its few toxicity to normal cells and potent anticancer activity in vitro and in vivo, honokiol might be a potential chemotherapy candidate in treating human colorectal carcinoma.
- Citation: Chen F, Wang T, Wu YF, Gu Y, Xu XL, Zheng S, Hu X. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol 2004; 10(23): 3459-3463
- URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3459
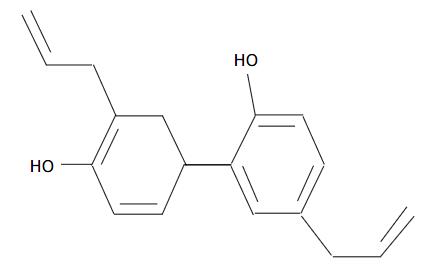
In traditional Chinese medicine, Houpu (Magnolia officinalis) has long been one of the important herbs. It is widely used by Chinese people in treating thrombotic stroke, typhoid fever, anxiety and nervous disturbance[1] when used in combination with other herbs. With its major active constituent extracted from the bark of Houpu, honokiol has been found having a variety of pharmacological effects, such as anti-inflammatory[2], antithrombotic[3], anti-arrhythmic[4], antioxidative[5] and anxiolytic effects[6]. Recently, honokiol has been reported to exhibit a potent cytotoxicity by inducing cell apoptosis in rat and human leukemia cells[7,8], human fibrosarcoma cells[9], human squamous lung cancer CH27 cells[10] and human SVR angiosarcoma cells[11], yet there has been no report on honokiol in the treatment of human colorectal carcinoma.
Previous studies have shown that honokiol can induce apoptosis with characteristic morphological changes and DNA fragments, involvement of Caspase family and Bcl-2 family[10]. It could reduce tumor volume of SVR angiosarcoma in nude mice[11]. However, it is still unclear whether honokiol can be used as a monomer in clinic. In this study, we chose colorectal carcinoma cells to investigate its possible application in clinical practice.
Human colorectal cell line RKO was provided by the Cancer Institute of Zhejiang University. Cells were maintained in RPMI-1640 medium (Gibco BRL) supplemented with 100 mL/L heat-inactivated fetal bovine serum (Si-Ji-Qing Biotechnology Co, Hangzhou, China), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a 50 mL/L CO2 atmosphere. Antibodies used in this study including Caspases-3, -9 and pan-actin were purchased from NeoMarkers, Fremont, CA, USA. Honokiol was obtained from the National Institute for Pharmaceutical and Biological Products, Beijing, China. The drug was dissolved in dimethyl sulfoxide (DMSO) at the stock concentration of 10 g/L. It was further diluted in culture medium at the final DMSO concentration < 1%. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Corporation, USA. Six weeks old female Balb/ c mice and female BALB/c nude mice (weighing 20 ± 2 g each) were provided by the Experimental Animal Center of Zhejiang University.
Cells (1 × 10 4 in 100 μL) were seeded on 96-well plates in triplicate. Following a 24-h- culture at 37 °C, the medium was replaced with fresh medium at various concentrations of honokiol in a final volume of 200 μL. Cells were incubated at 37 °C for 68 h[12,13]. Then 50 μL of MTT (2 mg/mL in PBS) was added to each well, incubated for an additional 4 h, the plate was centrifuged at 1000 r/min for 10 min, then the medium was removed. MTT formazan precipitate was dissolved in 100 μL of DMSO, shaken mechanically for 10 min and then read immediately at 570 nm in a plate reader (Opsys MR, Denex Technology, USA).
RKO cells were exposed to a variety of concentrations of honokiol for 24 h, then examined under reverse microscope (Olympus) and imaged with a digital camera. To detect DNA fragments, the cells were collected and lysed with lysis buffer containing 50 mmol/L Tris-HCL (pH 7.5), 20 mmol/L EDTA, and 10 g/L NP-40. Then 10 g/L SDS and RNase (5 μg/mL) were added to the supernatants, and incubated at 56 °C for 2 h, followed by incubation with proteinase K (2.5 μg/mL) at 37 °C for 2 h. After the DNA was precipitated by addition of both ammonium acetate (3.3 mol/L) and ethanol (99.5 %), it was dissolved in a loading buffer. DNA fragmentation was detected by electrophoresis on 15 g/L agarose gels and was visualized with ethidium bromide staining.
Honokiol-treated RKO cells and vehicles were fixed with 700 mL/L alcohol for 15 min at 4 °C, then stained with 1.0 μg/mL propidium dodide (PI, Sigma, USA). The red fluorescence of DNA-bound PI in individual cells was measured at 488 nm with a FACSCalibur (Becton Dickinson, USA) and the results were analyzed using ModFit 3.0 software. Ten thousand events were analyzed for each sample.
RKO cells (5 × 10 6) were lysed by 4 g/L trypsin containing 0.2 g/L EDTA, then collected after washed twice with phosphate-buffered saline (PBS, pH 7.4). Total protein extracts from the cells were prepared using cell lysis buffer [150 mmol/L NaCl, 0.5 mol/L Tris-HCL (pH 7.2), 0.25 mol/L EDTA (pH 8.0), 10 g/L Triton X-100, 50 mL/L glycerol, 12.5 g/L SDS]. The extract (30 μg) was electrophoresed on 120 g/L SDS-PAGE gel and electroblotted onto polyvinydene difluoride membrane (PVDF, Millipore Corp., Bedford, MA) for 2 h in a buffer containing 25 mmol/L Tris-HCL (pH 8.3), 192 mmol/L glycine and 200 mL/L methanol. The blots were blocked with 50 g/L nonfat milk in TBST washing buffer for 2 h at room temperature and then incubated at 4 °C overnight with anti-caspase-3 and -9 antibodies (NeoMarkers), all of which were diluted 1:400 in TBST. After washed at room temperature with washing buffer, they were labeled with peroxidase-conjugated secondary antibodies.
Primary human fibroblast cells were derived from fresh skin[13]. Human monocytes were isolated from umbilical blood by Ficoll-Hyaque separation method while seeded in 96-well microplates at the concentration of 5 μg/mL phytohemagglutinin (PHA)[14]. HUVECs isolated from fresh human umbilical cords were inoculated into 96-well microplates at 5000 cells/well[15]. Following treatment with the concentrations of 5, 10, 20, 40 μg/mL of honokiol for 24 h, cell viability was estimated by trypan blue exclusion. Three wells were measured at each time point/concentration. Six wells were measured for each concentration of test compound. All toxicity experiments were at least repeated three times.
For intraperitoneal (ip) pharmacokinetics study, honokiol was mixed with PEG400/dextrose by 7:3 in volume at a concentration of 20 g/L. Thirty Balb/c mice received honokiol by i.p. at a dose of 250 mg/kg. Blood samples were collected as described[16]. The plasma concentrations were tested by the total fluorescence intensity at 290 nm with high phase liquid chromatograph (HPLC, HEWLETT PACKARD)[17]. Chromatography was carried out using a Hypersil C18 column (5 mm × 100 mm × 2.1 mm) with a flow rate of 0.2 mL/min. Pharmacokinetic parameters were estimated by Modkine programs (Biosoft, UK).
Effect of honokiol on ascites formation in Balb/c nude mice Five Balb/c nude mice in each group were transplanted with 1 × 10 7 RKO cells by ip. Honokiol was dissolved in PEG400/ Tween 20 (9:1 by volume). Honokiol-treated group was intragastrically administered 2 mg of honokiol per mice on d 0, 2, 4, 6, 8 and 10 after inoculation of RKO cells. While the control group was given the same volume of PEG400/Tween20. Animals were regularly monitored for the appearance of peritoneal bulge and body weight.
Effect of honokiol on solid tumor growth in Balb/c nude mice RKO cells (5 × 10 6) were injected subcutaneously at the axilla of Balb/c nude mice. When tumors became visible about one week after implant, the animals were randomized into four groups: Adriamycin-treated, honokiol-treated, vehicle and control. All mice received ip injection on days 8-11, 14-17, 21-24 and 28-31. Each mouse of honokiol-treated group received 80 mg/(kg/d) of honokiol suspended in PEG400/dextrose (7:3 by volume) intraperitoneally, while vehicle given equivalent solvent of PEG400/dextrose. Adriamycin dissolved in saline was injected ip at a dose of 2 mg/kg. Mice of control group were given the same volume of saline. Tumor growth was monitored with calipers every other day, and tumor volume was calculated using the modified ellipsoid formula: A/6 × A × B 2, where A is the longer axis and B is the axis perpendicular to A (Figure 1)[18].
Values were given as mean ± SD. Statistical comparisons were made by Student’s t-test, and P < 0.05 was taken as significant.
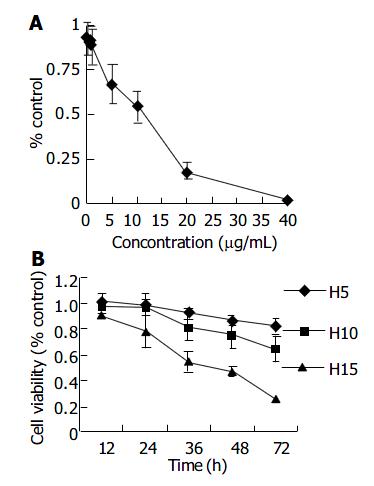
Cells treated with honokiol resulted in a dose- and time- dependent cytotoxicity in RKO cells. As shown in Figure 2A, honokiol-mediated cytotoxicity occurred at the concentration of 5 μg/mL and above. A significant decrease in cell number was seen at 10 μg/mL. The concentration leading to a 50% decrease in cell number (IC50) was about 12.47 μg/mL. Moreover, treatment of RKO cells with 5 μg/mL or 10 μg/mL of honokiol resulted in a significant growth inhibition at various time points (Figure 2B).
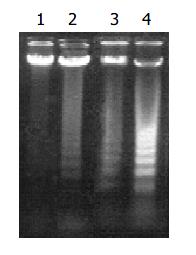
According to MTT results, we chose 5, 10, 15 μg/mL of honokiol to detect molecular changes. Under an inverted phase contrast microscope, honokiol-treated cells exhibited morphological features of apoptosis (Data not shown): rounded and granulated morphology, some vacuoles coming from cytoplasm, cell shrinkage and eventually detached from culture plates. In honokiol-treated cells, a degradation of chromosomal DNA into small internucleosomal fragments was evidenced by the formation of 180-200 bp DNA ladders on agarose gels (Figure 3), hallmark of cells undergoing apoptosis. No DNA ladders were detected in the samples isolated from control cultures. These results indicated that honokiol induced an apoptotic cell death in RKO cells.
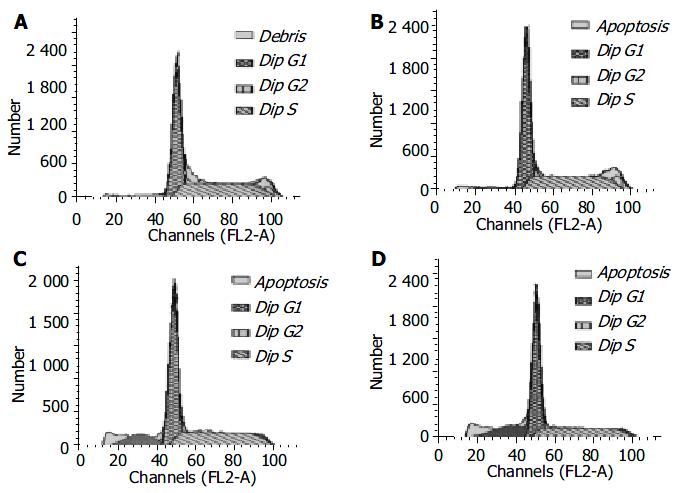
RKO cells were exposed to increasing concentrations of honokiol (5-15 μg/mL) for 48 h, and the growth of cells was analyzed with flow cytometry. In the absence of honokiol, the cell populations were at G1, S, and G2/M phases (Figure 4), accompanied with increased concentrations of honokiol by a concomitant increase of the G1 phase (Table 1). From Figure 4, the peak areas of subdiploid were enlarged with increased concentrations of honokiol. This observation led to a suggestion of G1 arrest. DNA fragmentation was seen when the cells were exposed to honokiol at 10 μg/mL and above (14.10% and 20.31%, respectively).
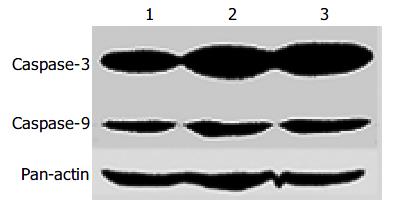
Since Caspases are the main factors in the apoptotic pathway, we investigated whether Caspases were involved in inducing apoptosis of RKO cells treated with honokiol. Cells induced for 48 h were analyzed for protein expression by Western blot. The results showed that Caspase-3 and -9 were up-regulated in a dose-dependent manner (Figure 5).
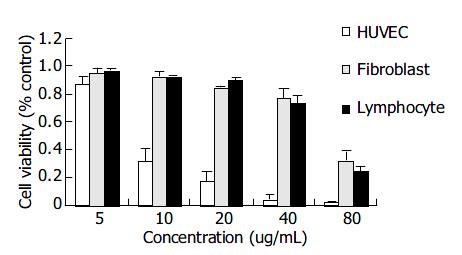
As shown in Figure 6, honokiol had little cytocidal effect on primary human fibroblast cells and human lymphocytes even up to 40 μg/mL. HUVEC cells after honokiol treatment resulted in a sharply dose-dependent cytotoxicity. These results demonstrated that human fibroblast cells and lymphocytes were more resistant to the honokiol-mediated cytotoxicity than HUVECs.
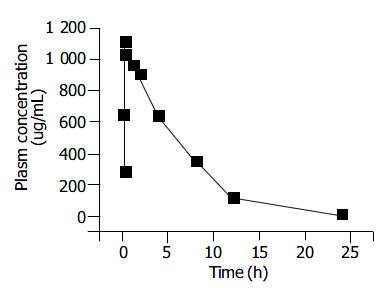
The pharmacokinetics of honokiol was evaluated after intraperitoneal injection of 250 mg/kg to BALB/c mice. The maximum plasma concentration of honokiol was observed at 27.179 ± 6.252 min after administration (Figure 7). The plasma disappearance curve could best be described by a first-order absorption one-compartment model, with an absorption half-life of 10.121 ± 2.761 min, and an elimination half-life of 5.218 ± 0. 461 h (Figure 7).
We studied the effect of honokiol on the growth of RKO tumor. There was significant inhibition of tumor growth by honokiol (Table 2). All the control mice developed peritoneal bulge and died by d 12. However, no peritoneal bulge was observed in 80% of the honokiol-treated animals and the mice survived for up to 30 d. These observations indicated the anti-tumor activity of honokiol in vivo (Table 2).
| Group | Ascites | MST(d) | Dead(D) | Living(L) | Survival percentage(L/(D + L) × 100%) |
| Control | 5 | 10.7 | 5 | 0 | 0% |
| Honokiol | 1 | 34.3 | 1 | 4 | 80% |
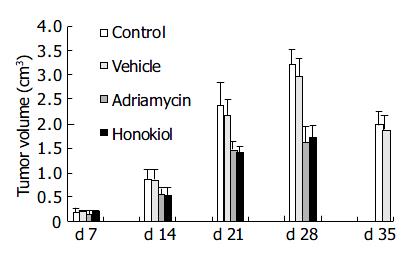
From Figure 8, animals in control and vehicle groups showed a progressive increase in tumor volume, with a growth rate of 1627.6% and 1408.2% respectively on d 28. While in treated groups, tumor growth rate was increased to 968.9% in adriamycin group and 709.9% in honokiol-treated group. There was a significant difference between honokiol-treated group and its control (treated with PEG400/dextrose) (P < 0.05). Similar results were found between adriamycin-treated group and its control (P < 0.05). These data further confirmed that honokiol had an effective anticancer activity in vivo.
The lifespan of mice in honokiol-treated group (80 mg/kg) was monitored and compared to the vehicle and adriamycin-treated group (Table 3). The mean survival time was 50.9 d in honokiol-treated group, with a significant prolongation compared to vehicle group (29.7 d, P < 0.05). The survival rate in honokiol-treated group was 176.7%, much higher than that in vehicle group (P < 0.01). There was no significant difference between control and vehicle groups and between adriamycin-treated and honokiol-treated groups. The results demonstrated that honokiol had a similar effect to adriamycin in prolongation of lifespan of tumor-bearing nude mice.
Many anticancer drugs kill tumor cells by inducing apoptosis. Previous studies suggest that growth inhibition by honokiol resulted from the induction of apoptosis in several cell lines[7-11]. A further study reported that in human squamous lung cancer cells, honokiol induced apoptosis by down-regulating Bcl-XL and sequentially activating Caspase cascade[10]. Our results confirmed this honokiol-mediated apoptotic progression in cultured RKO cells.
A variety of compounds with potent anticancer activity in vitro could not be used in clinic, one probable reason was due to their strong toxicities to normal cells[17,18]. Therefore, we first investigated honokiol’s effects on the toxicity of primary cultured human cells. Our data demonstrated that the IC50s were much higher in human fibroblasts and lymphocytes than in RKO cells, with the exception that HUVECs were more sensitive to honokiol. The safe doses for fibroblasts and lymphocytes could be up to 40 μg/mL (Figure 6), much greater than that for RKO and other tumor cells (data not shown). The phenomenon that primary cultured endothelial cells were more sensitive to honokiol might be related to the finding that honokiol had antiangiogenesis activity by inhibiting VEGF and its receptor 2 in vitro[11]. In chemotherapy, antiangiogenesis is another important target besides apoptosis induction in tumor cells. Based on the fewer toxicity to normal fibroblasts and lymphocytes, we thus proposed that honokiol could be a safe and potent candidate of chemotherapy for colorectal cancer in vivo.
Another major obstacle was that many potent agents were poorly absorbed and quickly cleared in vivo, such as curcumin. Curcumin was difficult to absorb in the gastrointestinal tract and, even when systemically administered, it was rapidly cleared by hepatic metabolism[11].
We then established two in vivo models with Babl/c nude mice bearing RKO cells, the ascitic tumor model and the solid tumor model. In RKO ascitic tumor model, honokiol exhibited a strong efficiency in prolonging the lifespan of ascitic tumor bearing mice and was highly effective on inhibiting intraperitoneal ascites in Balb/c nude mice. In our another model, honokiol also exhibited a potent efficiency in inhibiting solid tumor growth. In RKO incubated tumor bearing mice, honokiol at 80 mg/kg significantly inhibited the tumor growth and prolonged the lifespan compared to the vehicle (P < 0.05). The antitumor efficiency of honokiol was similar to that of the commonly used chemotherapy drug, adriamycin.
The results of the present study are encouraging because honokiol has shown significant inhibition of tumor growth in vitro and in vivo, as well as prolongation of lifespan in tumor-bearing mice in vivo. Together with its safety to human, honokiol might be a promising chemotherapy candidate in treating colorectal carcinoma or other cancers in clinic.
Co-first-authors: Fei Chen and Tao Wang
Edited by Wang XL Proofread by Zhu LH and Xu FM
| 1. | Squires RF, Ai J, Witt MR, Kahnberg P, Saederup E, Sterner O, Nielsen M. Honokiol and magnolol increase the number of [3H] muscimol binding sites three-fold in rat forebrain membranes in vitro using a filtration assay, by allosterically increasing the affinities of low-affinity sites. Neurochem Res. 1999;24:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur J Pharmacol. 2003;475:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Teng CM, Chen CC, Ko FN, Lee LG, Huang TF, Chen YP, Hsu HY. Two antiplatelet agents from Magnolia officinalis. Thromb Res. 1988;50:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Liou KT, Lin SM, Huang SS, Chih CL, Tsai SK. Honokiol ameliorates cerebral infarction from ischemia-reperfusion injury in rats. Planta Med. 2003;69:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Lo YC, Teng CM, Chen CF, Chen CC, Hong CY. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol. 1994;47:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Kuribara H, Kishi E, Hattori N, Yuzurihara M, Maruyama Y. Application of the elevated plus-maze test in mice for evaluation of the content of honokiol in water extracts of magnolia. Phytother Res. 1999;13:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Hibasami H, Achiwa Y, Katsuzaki H, Imai K, Yoshioka K, Nakanishi K, Ishii Y, Hasegawa M, Komiya T. Honokiol induces apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 1998;2:671-673. [PubMed] |
| 9. | Nagase H, Ikeda K, Sakai Y. Inhibitory effect of magnolol and honokiol from Magnolia obovata on human fibrosarcoma HT-1080. Invasiveness in vitro. Planta Med. 2001;67:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Yang SE, Hsieh MT, Tsai TH, Hsu SL. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem Pharmacol. 2002;63:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501-35507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Arbiser JL, Panigrathy D, Klauber N, Rupnick M, Flynn E, Udagawa T, D'Amato RJ. The antiangiogenic agents TNP-470 and 2-methoxyestradiol inhibit the growth of angiosarcoma in mice. J Am Acad Dermatol. 1999;40:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | LaMontagne KR, Moses MA, Wiederschain D, Mahajan S, Holden J, Ghazizadeh H, Frank DA, Arbiser JL. Inhibition of MAP kinase kinase causes morphological reversion and dissociation between soft agar growth and in vivo tumorigenesis in angiosarcoma cells. Am J Pathol. 2000;157:1937-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Beddy D, Watson RW, Fitzpatrick JM, O'Connell PR. Increased vascular endothelial growth factor production in fibroblasts isolated from strictures in patients with Crohn's disease. Br J Surg. 2004;91:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Goldrosen MH, Gannon PJ, Lutz M, Holyoke ED. Isolation of human peripheral blood lymphocytes: modification of a double discontinuous density gradient of Ficoll-Hypaque. J Immunol Methods. 1977;14:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Agrawal S, Jiang Z, Zhao Q, Shaw D, Cai Q, Roskey A, Channavajjala L, Saxinger C, Zhang R. Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: in vitro and in vivo studies. Proc Natl Acad Sci U S A. 1997;94:2620-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Tsai TH, Chou CJ, Cheng FC, Chen CF. Pharmacokinetics of honokiol after intravenous administration in rats assessed using high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994;655:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Eikesdal HP, Bjerkvig R, Dahl O. Vinblastine and hyperthermia target the neovasculature in BT(4)AN rat gliomas: therapeutic implications of the vascular phenotype. Int J Radiat Oncol Biol Phys. 2001;51:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |