Published online Nov 15, 2004. doi: 10.3748/wjg.v10.i22.3380
Revised: April 24, 2004
Accepted: April 29, 2004
Published online: November 15, 2004
AIM: To determine whether 2-(3-carboxy-1-oxopropy1) amino-2-deoxy-D-glucose (COPADG), a derivative of D-amino-glucose, inhibited the growth of human esophageal cancer cell line Eca-109.
METHODS: Effects of COPADG on Eca-109 cells cultured in RPMI 1640 medium were examined by a tetrazolium-based colorimetric assay (MTT assay).
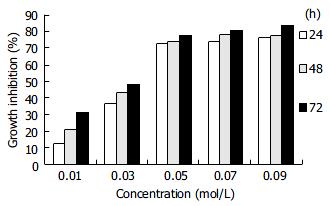
RESULTS: COPADG inhibited the growth of Eca-109 cells in a dose- and time-dependent manner; the maximum inhibition rate was 83.75%.
CONCLUSION: COPADG can directly inhibit the proliferation of Eca-109 cells, which may serve as the experimental evidence for development of new drugs for esophageal cancer therapy.
- Citation: Wu J, Lu H, Zhou Y, Qiao L, Ji R, Wang AQ, Liu WM, Xue QJ. Effect of 2-(3-carboxy-1-oxopropyl) amino-2-deoxy-D-glucose on human esophageal cancer cell line. World J Gastroenterol 2004; 10(22): 3380-3381
- URL: https://www.wjgnet.com/1007-9327/full/v10/i22/3380.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i22.3380
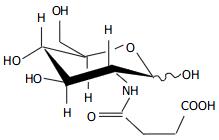
2-(3-carboxy-1-oxopropy1) amino-2-deoxy-D-glucose (COPADG, structure is shown in Figure 1) is a derivative of D-glucose, a monose derivative of degraded chitosan. Previous researches have discovered that some amino-D-glucose derivatives are capable of inducing leukemia K562 cells to differentiate into macrophages[1], but their efficacy in inducing apoptosis of tumor cells remains unclear. We conducted this study to determine whether COPADG could inhibit the proliferation of human esophageal cancer cell line Eca-109, to provide experimental evidence for new drug development for esophageal cancer therapy.
COPADG, synthesized by the Lanzhou Institute of Chemical Physics of Chinese Academy of Sciences, was dissolved in distilled water, filter-sterilized with 0.22 μm filter disc, and stored at 4 °C until use. Eca-109 cells were purchased from Shanghai Institute of Cell Biology of Chinese Academy of Sciences. RPMI 1640 medium, agarose, trypsin and fetal bovine serum (FBS) were obtained from Gibco BRL Company, and the reagents for MTT assay were purchased from Sigma Chemical Co. Ltd.
Eca-109 cells growing in logarithmic phase were cultured in RPMI 1640 medium supplemented with 100 mL/L heat-inactivated FBS, 100 μg/mL penicillin and 100 μg/mL streptomycin. The cells were maintained in a humidified atmosphere containing 50 mL/L CO2 at 37 °C. The medium was replaced every 48 h.
MTT assay[2] was based on the enzymatic reduction of the tetrazolium salt MTT in viable and metabolically active cells. Cells at 85% to 100% confluency were harvested with the mixture of 2.5 g/L trypsin and 0.2 g/L EDTA solution and seeded into a 96-well plate at a density of 4 × 103 /well, followed by incubation of the cells with COPADG at varied concentrations (0.01-0.09 mol/L) for different lengths of time (24-96 h, Table 1). The control cells were treated in the same way except that incubation was performed with sterile PBS instead of COPADG. After treatment, the medium was replaced by fresh medium and the cells were incubated for 4 h with 5 mg/mL MTT, which was dissolved in 150 μL of 100 g/L DMSO and kept for 1 h. The optical densities at 490 nm (A490nm) in the 96-well plates were determined using a microplate reader. Cell growth inhibition was estimated with the following formula: Growth inhibition (%) = 1 - A490nm (treated cells)/A490nm (control cells) × 100%.
| Concentration (mol/L) | A490nm | Inhibitory rate (%) | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0 (Control) | 1.505 ± 0.090 | 1.686 ± 0.067 | 1.745 ± 0.077 | 12.76 | 20.81 | 31.29 |
| 0.01 | 1.313 ± 0.053b | 1.336 ± 0.057b | 1.199 ± 0.083b | 12.76 | 20.81 | 31.29 |
| 0.03 | 0.958 ± 0.028 | 0.966 ± 0.086 | 0.900 ± 0.056 | 36.35 | 42.68 | 48.42 |
| 0.05 | 0.400 ± 0.064bc | 0.429 ± 0.065ba | 0.384 ± 0.050bc | 73.42 | 74.57 | 77.99 |
| 0.07 | 0.383 ± 0.045 | 0.360 ± 0.039 | 0.342 ± 0.037 | 74.55 | 78.66 | 80.44 |
| 0.09 | 0.355 ± 0.046c | 0.362 ± 0.053c | 0.280 ± 0.039a | 76.41 | 78.54 | 83.75 |
Results were expressed as mean ± SD. Each experiment was repeated at least three times. Statistical differences between each group were determined by single-factor analysis of variance and correlation analysis using SPSS 11.0 statistical software.
COPADG could effectively inhibit the growth of Eca-109 cells, the maximum inhibition rate was 83.75%. The inhibition exhibited an obvious time-and dose-dependent manner when the COPADG concentrations were below 0.05 mol/L, and higher concentrations tended to induce gradually stabilized inhibition, suggesting a saturation of the effects of COPADG (Table 1, Figure 2).
COPADG is a derivative of D-glucose, which is a low-molecular-weight compound with multiple biological activities and a monosaccharide derived from chitosan through release of an acetyl group followed by degradation of the residual group. D-glucose is the intermediate during the synthesis of protein-polysaccharide macromolecules, and distributed in almost every human tissue as a part of the structural components of cell membrane and tissues. From the biological standpoint, D-glucose not only is involved in hepatic and renal detoxification against toxic agents, but also acts to stimulate the anti-inflammatory response and enhance the synthesis of protein-polysaccharides. Studies[3,4] have also shown that D-glucose could inhibit tumor cell growth, and partial derivatives of D-glucose could potently induce differentiation of tumor cells. Some D-amine- glucose derivatives were able to induce leukemia K562 cells to differentiate into macrophages[5], but this effect failed to be observed in human hepatocellular carcinoma cell line[1]. Currently, COPADG has become a new focus of interest in cancer therapy.
By conducting this study, we aimed to test whether COPADG, the newest derivative of D-glucose, had any effect on the proliferation of human esophageal cancer cells. MTT assay showed that COPADG could effectively inhibit Eca-109 cell proliferation, in a marked time- and dose-dependent manner below the concentration of 0.05 mol/L; the maximum inhibition rate was 83.75%. The inhibition, however, became stable when the concentrations were higher than 0.05 mol/L, indicating that the effects of the drug might be saturated at this concentration.
The development of cancer has been considered to be the combined results of unrestricted cell proliferation and impairment of normal cell apoptosis[6]. These concepts provide a basis for the development of new strategies for cancer treatment. Agents with antiproliferative properties and proapoptotic effects have been widely investigated as potential chemotherapeutic options[7,8].
Conclusion, COPADG has obvious time- and concentration-dependent inhibitory effects against the proliferation of human esophageal cancer cell line Eca-109 in vitro, but whether this effect can be achieved in other cell lines still awaits further examination, which may also be necessary to clarify the mechanism underlying this effect.
Edited by Chen WW and Wang XL Proofread by Xu FM
| 1. | Wang Z, Qiao Y, Huang GS, Wang AQ, Zhang YQ, Feng JL, Yang GR, Guo Y, Liang R. Glucosamine and glucosamine hydrochloride induced leukemia cell line K562 differention into macrophage. Chin Pharmacological Bulletin. 2003;19:290-293. |
| 2. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38285] [Cited by in RCA: 39419] [Article Influence: 938.5] [Reference Citation Analysis (0)] |
| 3. | McDonnell TJ, Meyn RE, Robertson LE. Implications of apoptotic cell death regulation in cancer therapy. Semin Cancer Biol. 1995;6:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Xie QL, Dai Y, Sun FY, Lin J, Chen XY, Zhang MY, Chen XJ. The morphology of melanoma induced by glucosamine hydrochloride. Anhui Zhongyixueyuan Xuebao. 2002;21:42-44. |
| 5. | Wang Z, Qiao Y, Huang GS, Wang AQ, Zhang YQ, Feng JL, Yang GR, Guo Y, Liang R. Induction of macrophagic differentiation of leukemia cell line K562 by N-acetyl-D glucosamine. Disi Junyi Daxue Xuebao. 2003;24:46-48. |
| 6. | Oka Y, Naomoto Y, Yasuoka Y, Hatano H, Haisa M, Tanaka N, Orita K. Apoptosis in cultured human colon cancer cells induced by combined treatments with 5-fluorouracil, tumor necrosis factor-alpha and interferon-alpha. Jpn J Clin Oncol. 1997;27:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 10006] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 8. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |