Published online Nov 15, 2004. doi: 10.3748/wjg.v10.i22.3353
Revised: December 4, 2003
Accepted: December 8, 2003
Published online: November 15, 2004
AIM: To observe the protective effect of rhIL-1β on pancreatic islets of alloxan-induced diabetic rats.
METHODS: Protection of rhIL-1β on pancreatic islets of alloxan-induced diabetic rats (n = 5) was demonstrated with methods of immunohistochemistry and stereology. The concentration of serum glucose was measured by GOD method and that of serum insulin by RIA.
RESULTS: The concentration of serum glucose increased but that of insulin decreased after administration of alloxan(150 mg/kg), and the volume density and numerical density of the islets were zero. In rhIL-1β pretreated rats, although the concentration of serum insulin decreased (from 11.9 ± 3.0 mIU/L to 6.1 ± 1.6 mIU/L, P < 0.05), that of glucose was at normal level compared with the control group. As compared with alloxan group, the concentration of serum glucose in rhIL-1β pretreated rats decreased (from 19.4 ± 8.9 mmol/L to 12.0 ± 4.0 mmol/L, P < 0.05) and the volume density increased(0/L to. 1/L, P < 0.05).
CONCLUSION: rhIL-1β pretreatment may have protective effect on the islets of alloxan-induced diabetic rats.
- Citation: Wu LP, Chen LH, Zhang JS, Sun L, Zhang YQ. Protective effect of rhIL-1β on pancreatic islets of alloxan-induced diabetic rats. World J Gastroenterol 2004; 10(22): 3353-3355
- URL: https://www.wjgnet.com/1007-9327/full/v10/i22/3353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i22.3353
The cytokine interleukin-1β(IL-1β) can not only promote immunological reaction but also regulate neuro-endocrine system[1]. Previous studies found that IL-1β could stimulate the central adrenalin system, promote production of PG, and downregulate glucose metabolism[2].
Diabetes mellitus implicates many organs and tissues. It has been found that IL-1β decreases serum glucose in experimental animals and may potentially be therapeutic for diabetes mellitus[3,4]. In this experiment, we observed changes in serum glucose and insulin in alloxan induced diabetic rats treated with IL-1β. In addition, we detected the variation of volume density and numerical density of insulin positive pancreatic islets by ABC immunohistochemistry and stereology.
Twenty male Sprague-Dawley rats weighing 200 to 300 g were housed in a temperature-controlled room (24 ± 1 °C) with a 12-h light-dark cycle. The rats were provided with ordinary rat chow and water and divided into 4 groups (n = 5, every group): (1) Control group, each rat was injected with 2 mL saline every other day for 3 times, then injected with 2 mL saline on the 7th d; (2) rhIL-1β group, each rat was injected with 1 × 104 U rhIL-1β in 2 mL saline every other day for 3 times, then injected with 2 mL saline on the 7th d; (3) rhIL-1β pretreated group, each rat was injected with 1 × 104 U rhIL-1β in 2 mL saline every other day for 3 times, then was injected with 150 mg/kg alloxan in 2 mL saline on the 7th d; (4) Alloxan group, each rat was injected with 2 mL saline every other day for 3 times, then injected with 150 mg/kg alloxan in 2 mL saline on the 7th d.
Guinea pig anti-rat insulin antibody and SPA-HRP were prepared by Professor Yun-Long Zhu (Department of Physiology) and Professor Cai-Fang Xue (Department of Parasitology) of our university respectively. DAB was purchased from Sigma.
Forty-eight hours after last injection of alloxan or saline, rats were anesthetized with ether and sacrificed by cervical dislocation. The blood was collected into heparinised tubes (50 kU/L) and centrifuged (3000 g, 10 min, at room temperature). Plasma was aspirated and stored at -70 °C until assayed as described below. The pancreas was also removed and fixed in Bouin’s solution overnight. Each piece was embedded in paraffin and 4-μm sections were prepared.
Four-micrometer sections from rat pancreas were employed for immunohistochemical analysis. Several dilutions of the antibody were tested to find the optimal staining concentration before the entire series was processed. The staining procedure was carried out as previously reported, but without protease treatment. Briefly, (1) the sections were deparaffinized in xylene, hydrated in ethanol, and blocked with 3 mL/L H2O2 in methanol for 30 min to remove endogenous peroxides, then treated with 30 mL/L normal goat serum for 40 min and rinsed in 0.01 mol/L PBS. (2) The sections were incubated at 4 °C for 24 h with primary antibody, guinea pig anti-rat insulin antibody (1:1000 dilution, final concentration 5 mg/L); (3) then with secondary antibody, SPA-HRP (1:200 dilution), at room temperature for 1 h. (4) Peroxidative reaction was performed using DAB as chromogen. The sections were washed three times for 10 min after incubation. All slides were stained at the same time and under identical conditions. Primary antibodies were replaced by irrelevant antibodies and normal guinea pig serum as specific antibody control. Primary antibody was replaced by PBS as negative control. Primary antibody was omitted as blank control.
The concentration of serum glucose was measured by routine GOD method[5] and the concentration of serum insulin was measured by RIA[6,7]. Every sample was measured three times and the results were displayed as mean ± SD.
Five specimens from each group were used for morphometric analysis of slides processed for light microscopy. Two sections from each specimen were then selected and five different regions of each section were chosen for the measurement of volume density and number density by double blind method.
The results were calculated by the following formula; Nv = 2/3 ×π× NA × U (AT × A); Vv = A/AT. Data were analyzed by χ2 test. A P value of less than 0.05 was considered statistically significant.
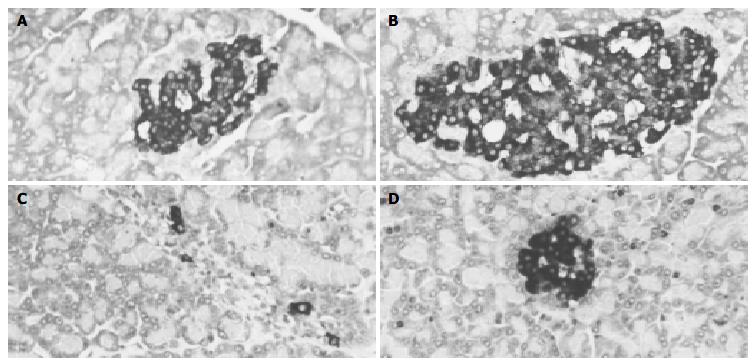
There were more insulin immunoreactive cells in pancreas of control group and rhIL-1β group than in alloxan group. Immunoreactive cells were mainly located in the central region of the pancreas. Insulin immunopositive cells had dark-brown reaction products in the cytoplasm mostly and nuclei were not stained (Figure 1A, B). The number of insulin immunopositive cells in alloxan group decreased remarkably and there were only a few positive cells in each pancreatic islet (Figure 1C). The number of insulin immunopositive cells in rat pancreas of rhIL-1β pretreated group decreased slightly compared with that of control group and rhIL-1β group, whereas, the number of insulin immunopositive cells in rat pancreas of rhIL-1β pretreated group increased remarkably compared with that of alloxan group(Figure 1D).
The concentration of serum glucose increased significantly but that of insulin decreased remarkably after administration of alloxan (150 mg/kg) for 48 h compared with those of control rats. At the same time, the volume density and numerical density of the islets were zero (Table 1).
Compared with control group rats, the concentration of serum insulin in rhIL-1β group rats increased significantly whereas that of glucose was at normal level. Immunohistochemistry and stereology data showed that there were no significant differences in the number density and volume density of the pancreatic islets between rhIL-1β group rats and control rats (Table 1).
In rhIL-1β pretreated group, when the rats were injected with alloxan for 48 h, although the concentration of serum insulin decreased significantly, that of glucose was at normal level compared with control group rats. Immunohistochemistry and stereology data showed that there were no significant differences in the number density of the pancreatic islets between rhIL-1β pretreated rats and control rats, whereas the volume density decreased markedly in rhIL-1β pretreated group (Table 1).
Interleukin 1β(IL-1β) is a multi-functional cytokine synthesized mainly by mononuclear/mo cells and is a key factor in the cytokine network[8,9]. IL-1β has many biological functions[9-21]. Previous study showed that IL-1β could decrease the serum glucose level and might be potentially a new drug for diabetes therapy[2-4]. In our experiment, when the rats were injected with alloxan (150 mg/kg) for 48 h, the concentration of serum glucose increased significantly, and that of insulin decreased remarkably. At the same time immunohistochemistry and stereology data showed that the value of number density and volume density of the pancreatic islets were zero. In rhIL-1β pretreated group, when the rats were injected with alloxan for 48 h, although the concentration of serum insulin decreased significantly, that of glucose was at normal level compared with control group rats. Immunohistochemistry and stereology data showed that there were no significant differences in the number density of the pancreatic islets between rhIL-1β pretreated rats and control rats, whereas the volume density decreased remarkably in rhIL-1β pretreated rats. Our results suggest that rhIL-1β has protective effect on pancreatic islets of alloxan-induced diabetic rats and provide the experimental evidence that rhIL-1β may be a new therapeutic drug for diabetes.
Co-correspondents: Li-Hua Chen
Edited by Zhu LH Proofread by Xu FM
| 1. | Song Y, Shi Y, Ao LH, Harken AH, Meng XZ. TLR4 mediates LPS-induced HO-1 expression in mouse liver: role of TNF-alpha and IL-1beta. World J Gastroenterol. 2003;9:1799-1803. [PubMed] |
| 2. | Lee SH, Woo HG, Baik EJ, Moon CH. High glucose enhances IL-1beta-induced cyclooxygenase-2 expression in rat vascular smooth muscle cells. Life Sci. 2000;68:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Ikeda U, Shimpo M, Murakami Y, Shimada K. Peroxisome proliferator-activated receptor-gamma ligands inhibit nitric oxide synthesis in vascular smooth muscle cells. Hypertension. 2000;35:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Doxey DL, Cutler CW, Iacopino AM. Diabetes prevents periodontitis-induced increases in gingival platelet derived growth factor-B and interleukin 1-beta in a rat model. J Periodontol. 1998;69:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Nakashima E, Nakamura J, Hamada Y, Koh N, Sakakibara F, Hotta N. Interference by gliclazide in the glucose oxidase/peroxidase method for glucose assay. Diabetes Res Clin Pract. 1995;30:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Durant S, Alves V, Coulaud J, Homo-Delarche F. Nonobese diabetic (NOD) mouse dendritic cells stimulate insulin secretion by prediabetic islets. Autoimmunity. 2002;35:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Amel Kashipaz MR, Swinden D, Todd I, Powell RJ. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clin Exp Immunol. 2003;132:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Musabak U, Bolu E, Ozata M, Oktenli C, Sengul A, Inal A, Yesilova Z, Kilciler G, Ozdemir IC, Kocar IH. Gonadotropin treatment restores in vitro interleukin-1beta and tumour necrosis factor-alpha production by stimulated peripheral blood mononuclear cells from patients with idiopathic hypogonadotropic hypogonadism. Clin Exp Immunol. 2003;132:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Hasegawa K, Ichiyama T, Isumi H, Nakata M, Sase M, Furukawa S. NF-kappaB activation in peripheral blood mononuclear cells in neonatal asphyxia. Clin Exp Immunol. 2003;132:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Tsirpanlis G, Chatzipanagiotou S, Ioannidis A, Ifanti K, Bagos P, Lagouranis A, Poulopoulou C, Nicolaou C. The effect of viable Chlamydia pneumoniae on serum cytokines and adhesion molecules in hemodialysis patients. Kidney Int Suppl. 2003;84:S72-S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Li L, Jacinto R, Yoza B, McCall CE. Distinct post-receptor alterations generate gene- and signal-selective adaptation and cross-adaptation of TLR4 and TLR2 in human leukocytes. J Endotoxin Res. 2003;9:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Allan SM, Pinteaux E. The interleukin-1 system: an attractive and viable therapeutic target in neurodegenerative disease. Curr Drug Targets CNS Neurol Disord. 2003;2:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci. 2003;44:4097-4104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, Rothwell NJ. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci. 2003;23:6470-6474. [PubMed] |
| 17. | Boutin H, Kimber I, Rothwell NJ, Pinteaux E. The expanding interleukin-1 family and its receptors: do alternative IL-1 receptor/signaling pathways exist in the brain. Mol Neurobiol. 2003;27:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Lu M, Zhang M, Kitchens RL, Fosmire S, Takashima A, Munford RS. Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J Exp Med. 2003;197:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Wheeler RD, Brough D, Le Feuvre RA, Takeda K, Iwakura Y, Luheshi GN, Rothwell NJ. Interleukin-18 induces expression and release of cytokines from murine glial cells: interactions with interleukin-1 beta. J Neurochem. 2003;85:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |