Published online Nov 15, 2004. doi: 10.3748/wjg.v10.i22.3261
Revised: February 4, 2004
Accepted: March 2, 2004
Published online: November 15, 2004
AIM: To investigate the expression of vascular endothelial growth factor-C (VEGF-C) and the relationship between VEGF-C and lymphangiogenesis, lymph node metastasis in colorectal cancer.
METHODS: Fifty six cases of colorectal cancer were selected randomly. Expression of VEGF-C was detected by immuno- histochemistry, and lymphatic vessels were stained by enzyme histochemical method.
RESULTS: VEGF-C expression was found in 66.7% (37/56) patients. In VEGF-C positive and negative patients, the lymphatic vessel density was 25.16 ± 7.52 and 17.14 ± 7.22, respectively (P < 0.05). The rate of lymph node metastasis in VEGF-C positive patients (81.1%) was significantly higher than that in the negative group (42.1%).
CONCLUSION: VEGF-C expression may induce lymphangiogenesis in colorectal cancer, as a result, tumor cells can entry the lymphatic vessels easily. VEGF-C may serve as a useful prognotic factor in colorectal carcinoma.
- Citation: Jia YT, Li ZX, He YT, Liang W, Yang HC, Ma HJ. Expression of vascular endothelial growth factor-C and the relationship between lymphangiogenesis and lymphatic metastasis in colorectal cancer. World J Gastroenterol 2004; 10(22): 3261-3263
- URL: https://www.wjgnet.com/1007-9327/full/v10/i22/3261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i22.3261
Colorectal cancer is a common cause of death throughout the world including China. The lymphatic system is the primary pathway of metastasis for gastrointestinal tract malignancies, and the extent of lymph node involvement is a key prognostic factor for the outcome of patients. However, the mechanism of lymphatic metastasis remains unclear.
Lymphangiogenesis, the development of new lymph vessels, is a relatively new area of clinical investigations. Recently, vascular endothelial growth factor C (VEGF-C) has been identified as a new member of the VEGF family[1,2], and is belived to be the only lymphangiogenic factor in the VEGF family. It activates both vascular endothelial growth factor receptor 2 (VEGFR-2) and VEGFR-3[3,4]. VEGF-C induces the proliferation of lymphatic vessels in the stroma of gastric carcinoma by activating VEGFR-3 in lymphatic endothelial cells[5]. But the precise role of VEGF-C in colorectal cancer has not been clearly understood. Therefore, in the current study, we used an enzyme-histochemical method for 5’-nucleotidase (5’-Nase) to distinguish lymph vessels in colorectal carcinoma, and immunohistochemistry to examine the correlation between the expression of VEGF-C, lymphangiogenesis and the clinicopathologic features.
Fifty-six Chinese colorectal carcinoma patients were surgically treated in the Department of Surgery, the Forth Hospital of Hebei Medical University, China from 2001 to 2002. The patients included 34 males and 22 females, and ranged in age from 33 to 75 years (average age 58.5 years). The lesions included 13 colon cancers and 43 rectal cancers, two patients with stage I, 17 patients with stage II, 34 patients with stage III and three patients with stage IV.
Immunohistochemical staining for VEGF-C was performed using the streptavidin-peroxidase technique. Formalin fixed and paraffin embeded tissues were cut into 4 μm thick sections and placed on saline coated slides. After deparaffinization in xylene and rehydration, endogeneous peroxidase activity was blocked after incubated with 30 mL/L hydrogen peroxidase for 20 min. Tissue sections were then autoclaved at 121 °C in 10 mmol/L citrate buffer (pH6.0) for 10 min for antigen retrieval and cooled at room temperature for 30 min, them incubated for 3 h with a 1:40 dilution of anti-VEGF-C rabbit polyclonal antibody (Santa Cruz Biotech, USA). Bound peroxidase was visualized using a solution of diaminobezidine as chromogen, and nuclei were counterstained with hematoxylin. Scoring was carried out by two independent observers who were blinded to the patient’s status. Positive staining was defined as the presence of VEGF-C immunoreactivity in at least 10% of tumor cells.
Cryosections (7 μm thick) of tissue were processed for 5’-nucleotide-alkaline phosphatase (5’-Nase-ALPase) double staining according to Ji et al[6]’s report. After rinsed in 0.1 mol/L cacodylate buffer (pH7.2), specimens were incubated in the reaction medium for 5’-Nase for 50 min at 37 °Cwith 5’-adenosine monophosphate (AMP) (Sigma chemical, St. Louis, MO, USA) as a substrate, lead nitrate (Sigma, USA) as a capture agent, and 2 mmol/L L-tetramisole (Sigma, USA) as an inhibitor of nonspecific alkaline phosphatase. After washed with distilled water, the tissues were treated with 10 mg/L ammonium sulfide solution for 1 min at room temperature. Then, the sections were incubated in the reaction medium for ALPase for 30 min at 4 °C with fast blue BB, N,N-dimethylformamide, naphtol AS-MX phosphate (Sigma, USA).
The stained sections were screened at × 40 magnification to identify the regions of the highest vascular density within the tumor. Lymphatic vessels were counted in 3 regions of the highest vascular density at × 100 magification. The number of lymphatic vessels was the mean of vessels in these areas.
The data were analysed by t test and χ2 test. All reported P values were two-sided, and P < 0.05 was considered statistically significant.
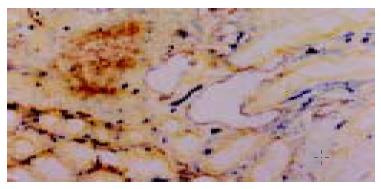
In specimens of normal colorectal mucosa, no VEGF-C protein was stained. Among the 56 examined tumors, 37 (66.7%) showed VEGF-C protein expression in the cytoplasm (Figure 1).
Lymphatic vessels were 5’-Nase positive (brown) and blood vessels were ALPase positive (blue). The most lymphatic vessels were enlarged and dilated especially in peritumor areas. The walls of lymphatic vessels were thinner than that of blood vessels, and their profiles of lumens were more irregular. While the intratumoral lymphtic vessels were strip-like (Figure 2). The LVD of VEGF-C positive tumors (25.16 ± 7.52) was significantly higher than that of VEGF-C negative tumors (17.14 ± 7.22) (P = 0.04). No significant lymphangiogenesis was observed in normal and adenoma tissues.
VEGF-C expression was observed frequently in patients with colorectal carcinoma. A positive association between VEGF-C expression and lymphatic metastasis was observed (P < 0.05, Table 1). However, no significant correlations with gender, histologic differentiation, invasive depth, TNM stage were observed.
| Characteristic | n | VEGF-C | P | ||
| (+) | (-) | ||||
| Male | 34 | 25 | 9 | ||
| Gender | 0.143 | ||||
| Female | 22 | 12 | 10 | ||
| Lymph node | |||||
| (+) | 37 | 30 | 8 | ||
| metastasis | 0.007 | ||||
| (-) | 9 | 8 | 11 | ||
| Well | 40 | 25 | 15 | ||
| Differentiation | 0.372 | ||||
| Poorly | 16 | 12 | 4 | ||
| Muscularis | 17 | 10 | 7 | ||
| Invasion depth | 0.499 | ||||
| Adventitia | 39 | 27 | 12 | ||
| I-II | 19 | 13 | 6 | ||
| Stage | 0.790 | ||||
| III-IV | 37 | 24 | 13 | ||
Clinical and pathological data pointed to the metastasis of solid tumors via the lymphatics as an important early event in metastatic diseases[7]. However, little has been achieved in lymphangiogenesis and the role of lymphangiogenesis in promoting the metastasis of tumor cells via the lymphatic vessels. This might, in part, be due to difficulty in studing lymphatic vessels because of their morphology and lack of lymphatic-specific markers[6,7]. Approximatly 10 years ago, Kato et al[8] developed an enzyme-histochemical method for 5’-Nase-ALPase to distinguish lymphatic vessels from blood vessels. Many other markers such as LYVE1 and podoplanin were found later[9,10]. Now, lymphangiogenesis or new lymphatic vessel growth has become an exciting area of reaserch in cancer biology[11]. So far, the occurrence and involvment of lymphangiogenesis have been demonstrated in some experimental mouse tumors , human head and neck cancers, oral squamous cell cancer by using these markers[12-16]. Similarly, in our study, lymphangiogenesis was observed in primary colorectal carcinomas, and the vicinity of tumor was the dominant region. Most of lymphatic vessels were dilated, as a result, tumor cells could invade the lymphatics easily. On the other hand, most of the intratumor lymphatic capillaries were strip-like. It is believed that tumor cells could utilize peritumoral lymphatics to spread, while intratumoral lymphatics should be regarded as an additional pathway rather than a necessity for metastasis[17].
Vascular endothelial growth factor-C (VEGF-C) is the first lymphangiogenic factor identified. Moreover, there is ample evidence for the expression of VEGF-C in human tumors. But the precise role of VEGF-C in colorectal cancer is less well understood. With respect to VEGF-C expression, several authors have demonstrated associations between this growth factor expression and poor clinicopathological outcome[18-25]. Immuno- histochemical detection of VEGF-C expression at the deepest invasive site of colorectal carcinoma was found in about 50% advanced tumors. Furudoi et al[26] suggested that the expression of VEGF-C was correlated with lymphatic and venous invasion, lymph node status, Dukes stage, liver metastasis, depth of invasion, poorer histological grade and microvessel density.
It is widely known that VEGF-C can bind to both VEGFR-2 and VEGFR-3. Activation of VEGFR-2 results in the mitogenesis of vascular endothelial cells. In contrast, VEGFR-3 activation by VEGF-C is considered to induce proliferation of lymphatic endothelial cells. Thus, angiogenic versus lymphangiogenic responses to VEGF-C have been found to depend on the expression of its receptors in blood versus lymphatic endothelial cells of the target tissue[27]. In addition, the activation of lymphatics by VEGF-C is considered to induce secretion of chemokines and similar factors by the lymphatic endothelium, thus attracting tumor cells and facilitating their entry into lymphatics[28]. Besides VEGF-C, another new member of VEGF family, VEGF-D, could also stimulate lymphangiogenesis by activating VEGFR-3 in human tumors[29-32]. But the relationship between VEGF-C and VEGF-D expressions, as well as the role of VEGF-D in human tumors is still unclear.
In summary, our findings demonstrate a causal role of lymphangiogenesis in tumor metastasis, suggesting VEGF-C expression is related to the high incidence of metastasis in colorectal carcinoma. However, the mechanism of lymphangiogenesis is extremely complex, which is a subject of ongoing investigation.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality. Clin Cancer Res. 2001;7:462-468. [PubMed] |
| 2. | Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 586] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Baldwin ME, Stacker SA, Achen MG. Molecular control of lymphangiogenesis. Bioessays. 2002;24:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, Sugiyama K, Partanen T, Yamamoto H, Sasaki T. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 2001;37:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Ji RC, Kato S. Lymphatic network and lymphangiogenesis in the gastric wall. J Histochem Cytochem. 2003;51:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Kato S. Intralobular lymphatic vessels and their relationship to blood vessels in the mouse thymus. Light- and electron-microscopic study. Cell Tissue Res. 1988;253:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med. 2003;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Reis-Filho JS, Schmitt FC. Lymphangiogenesis in tumors: what do we know. Microsc Res Tech. 2003;60:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Duff SE, Li C, Jeziorska M, Kumar S, Saunders MP, Sherlock D, O'Dwyer ST, Jayson GC. Vascular endothelial growth factors C and D and lymphangiogenesis in gastrointestinal tract malignancy. Br J Cancer. 2003;89:426-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Härkönen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713-722. [PubMed] |
| 14. | He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 376] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315-1320. [PubMed] |
| 16. | Sedivy R, Beck-Mannagetta J, Haverkampf C, Battistutti W, Hönigschnabl S. Expression of vascular endothelial growth factor-C correlates with the lymphatic microvessel density and the nodal status in oral squamous cell cancer. J Oral Pathol Med. 2003;32:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Cassella M, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2002;979:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Tanaka K, Sonoo H, Kurebayashi J, Nomura T, Ohkubo S, Yamamoto Y, Yamamoto S. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma. Clin Cancer Res. 2002;8:1125-1131. [PubMed] |
| 19. | Takahashi A, Kono K, Itakura J, Amemiya H, Feng Tang R, Iizuka H, Fujii H, Matsumoto Y. Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology. 2002;62:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol. 2001;78:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Masood R, Kundra A, Zhu S, Xia G, Scalia P, Smith DL, Gill PS. Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops. Int J Cancer. 2003;104:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Kaio E, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Clinical significance of angiogenic factor expression at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2003;64:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004;10:352-355. [PubMed] |
| 26. | Furudoi A, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Chayama K, Shimamoto F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Nisato RE, Tille JC, Pepper MS. Lymphangiogenesis and tumor metastasis. Thromb Haemost. 2003;90:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 715] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 29. | Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Flt-4-positive vessel density correlates with vascular endothelial growth factor-d expression, nodal status, and prognosis in breast cancer. Clin Cancer Res. 2003;9:5313-5317. [PubMed] |
| 30. | Stacker SA, Hughes RA, Achen MG. Molecular targeting of lymphatics for therapy. Curr Pharm Des. 2004;10:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Koyama Y, Kaneko K, Akazawa K, Kanbayashi C, Kanda T, Hatakeyama K. Vascular endothelial growth factor-C and vascular endothelial growth factor-d messenger RNA expression in breast cancer: association with lymph node metastasis. Clin Breast Cancer. 2003;4:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |