Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3165
Revised: April 9, 2004
Accepted: April 16, 2004
Published online: November 1, 2004
AIM: To investigate the curative effects of oral and nasal administration of chicken type II collagen (CII) on adjuvant arthritis (AA) in rats with meloxicam-induced intestinal lesions.
METHODS: AA model in Sprague-Dawley (SD) rats with or without intestinal lesions induced by meloxicam was established and those rats were divided randomly into six groups which included AA model, AA model + meloxicam, AA model + oral CII, AA model + nasal CII, AA model + meloxicam + oral C II and AA model + meloxicam + nasal CII (n = 12). Rats was treated with meloxicam intragastrically for 7 d from d 14 after immunization with complete Freund’s adjuvant (CFA), and then treated with chicken CII intragastrically or nasally for 7 d. Histological changes of right hind knees were examined. Hind paw secondary swelling and intestinal lesions were evaluated. Synoviocyte proliferation was measured by 3-(4,5-dimethylthiazol-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) method. Activities of myeloperoxidase (MPO) and diamine oxidase (DAO) from supernatants of intestinal homogenates were assayed by spectrophotometric analysis.
RESULTS: Intragastrical administration of meloxicam (1.5 mg/kg) induced multiple intestinal lesions in AA rats. There was a significant decrease of intestinal DAO activities in AA + meloxicam group (P < 0.01) and AA model group (P < 0.01) compared with normal group. DAO activities of intestinal homogenates in AA + meloxicam group were significantly less than those in AA rats (P < 0.01). There was a significant increase of intestinal MPO activities in AA + meloxicam group compared with normal control (P < 0.01). Oral or nasal administration of CII (20 μg/kg) could suppress the secondary hind paw swelling(P < 0.05 for oral CII; P < 0.01 for nasal CII), synoviocyte proliferation (P < 0.01) and histopathological degradation in AA rats, but they had no significant effects on DAO and MPO changes. However, oral administration of CII (20 μg/kg) showed the limited efficacy on arthritis in AA + meloxicam model and the curative effects of nasal CII (20 μg/kg) were shown to be more efficient than that of oral CII (20 μg/kg) both in AA model and in AA + meloxicam model (P < 0.05).
CONCLUSION: Oral administration of CII shows the limited efficacy on arthritis in AA rats with intestinal lesions, and nasal administration of CII is more efficient than oral administration of CII to induce mucosal tolerance in AA rats.
- Citation: Zheng YQ, Wei W, Shen YX, Dai M, Liu LH. Oral and nasal administration of chicken type II collagen suppresses adjuvant arthritis in rats with intestinal lesions induced by meloxicam. World J Gastroenterol 2004; 10(21): 3165-3170
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3165
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of the joints including proliferation of the synovium and progressive erosion of cartilages and bones[1]. The goals of treatment include reduction of pain and inflammation, maintenance of functional ability, slowing of disease progression, and prevention of adverse effects of drugs[2]. Administering antigens via a mucosal route has been recognized as a means to induce tolerance. The phenomenon of oral tolerance (OT) was first reported in 1911 by Wells[3]. Further studies demonstrated that CII could suppress arthritis induced by adjuvant[4], antigen[5], pristane[6] and collagen[7] in mice and rats. Moreover, several clinical trials based on the results from those experimental animal systems, have been conducted to test the feasibility of using oral tolerance in the treatment of RA[8,9]. However, it was reported that oral administration of CII in a low dose of 10 μg for 10 times demonstrated a doubtful effect on murine collagen-induced arthritis (CIA) model[10]. In clinical trials, oral administration of CII showed no efficacy in human arthritis given along with existing treatment[11] and moreover, the Peyer’s patches (pp) in the gut-associated lymphoid tissue (GALT) were considered to mediate oral tolerance[12]. As we know, nonsteroidal anti-inflammatory drugs (NSAIDs) including meloxicam, which have been usually considered as the main drugs in the management of RA, could induce digestive lesions[13,14], and it might be an important reason for the invalidation of oral administration of CII. Therefore, nasal administration of CII should be considered as an alternative.
Adjuvant arthritis (AA) in rats is an experimental model that shares some features with human RA, such as swelling, cartilage degradation and loss of joint function[15]. In the present study, therefore, AA rats with or without intragastrically administration of meloxicam were used to compare the curative effects of oral and nasal administration of CII in rats with or without intestinal lesions. Based on the results of our report that oral administration of CII suppressed pro-inflammatory mediator production by synoviocytes in rats with adjuvant arthritis from 5 to 500 μg/kg[12], we chose the single dose of 20 μg/kg in the present study.
Diamine oxidase (DAO) is an intracellular enzyme with a high activity existing in intestinal villous cells and can catalyze the oxidation of diamines such as histamine, putrescine and cadaverine in both human beings and all other mammalians. The activity of DAO in intestinal mucosa decreases when its cells are injured. Thus the determination of the DAO activity of intestinal mucosa can reflect the changes in its cellular integrity[16]. Myeloperoxidase (MPO) plays a fundamental role in oxidant production by neutrophils and is considered to be a major effector in the tissue damage[17]. In our studies, MPO and DAO were used as markers of intestinal dysfunction.
The purpose of the present study was to compare the curative effects of oral and nasal administration of CII in AA rats with or without intestinal lesions induced by meloxicam.
Male Sprague-Dawley (SD) rats weighting 147 ± 25 g were purchased from Shanghai BK Experimental Animal Center (Grade II, Certificate No D-65). All rats were housed, five per cage and fed a standard laboratory chew and water and kept on a 12 h dark/12 h light cycle at a constant temperature of 20°C ± 5 °C. All experimental protocols described in this study were approved by the Ethics Review Committee for Animal Experimentation of Institute of Clinical Pharmacology, Anhui Medical University.
Meloxicam (batch number 020401), nasal chicken CII (batch number 00031004) and oral chicken CII (batch number 00031002) were obtained from Shanghai Institute of Herb and Bio-medical Engineering and dissolved in sterile water containing 5 g/L carboxymethylcellulose (CMC-Na) solution. Dimethyl sulfoxide (batch number 000601) was obtained from Shanghai Vitriolic Factory. Bacillus Calmette Guerin (BCG) was obtained from Shanghai Biochemical Factory. Collagenase type II, trypsin, 3-(4,5-dimethylthiazol-2-thiazolyl)-2,5- diphenyl-2H tetrazolium bromide (MTT), RPMI 1640 medium, lipopolysaccharide (LPS), horseradish perozidase, cadaverine dihydrochloride, diamine oxidase (DAO) and O-dianisidine were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Complete Freund’s adjuvant (CFA) was prepared by suspending heat-killed BCG in liquid paraffin at 10 mg/mL. AA was induced by a single intradermal injection of 100 μL of CFA into the left hind paw. On d 0, 14, 16, 20, 24 and 28 after immunization, the right hind paw volume was measured with a water replacement plethsmometer (Mukomachi Kiai CD, Japan). Paw swelling (mL) was calculated by taking away the paw volume on d 0 from the related one on d 14, 16, 20, 24 and 28.
The rats with AA were divided randomly into six groups which included AA model, AA model + meloxicam, AA model + oral CII, AA model + nasal CII, AA model + meloxicam + oral C II and AA model + meloxicam + nasal C II. From d 14 after immunization, the rats were fasted for 12 h and then treated with meloxicam (1.5 mg/kg body weight) intragastrically for 7 d. The rats in normal group, AA group, AA model + oral CII group and AA + nasal CII group, were treated with an equal amount of vehicle. From d 21 after immunization, the rats were treated with oral or nasal administration of CII (20 μg/kg body mass) for 7 d. The rats in normal group, AA group and AA + meloxicam group, were treated with an equal amount of vehicle.
Rats were sacrificed on day 29 after immunization. The small intestines were removed, spread out on filter paper and opened by a longitudinal incision along the antimesenteric side. The length and width of each lesion were observed and the extent of haemorrhage was also observed according to scale scores 0-2: 0, absence; 1, slight haemorrhage; 2, severe haemorrhage.
Synovial tissue from rat knees was excised and dispersed with sequential incubation of 4 g/L collagenase type II and 2.5 g/L trypsin and then synoviocytes were suspended in RPMI-1640 medium with 200 mL/L fetal bovine serum (FBS) at a concentration of 1 × 10 6 cells/mL. In 96-well plates, 100 μL of synoviocyte suspension (1 × 10 5 cells/well) was cultured in triplicate with 100 μL of LPS (10 mg/L) in RPMI-1640 medium (pH7.0) containing 200 mL/L heat-inactivated FBS, 100 U/mL penicillin, 100 g/L streptomycin, 2 mmol/L L-glutamine , 5 × 10 -5 moL/L 2-mercaptoethanol and 25 mmol/L HEPES. After cultured for 48 h at 37 °C in humidified atmosphere containing 50 mL/L CO2 in air, proliferation of synoviocytes was measured by the MTT colorimetric method[18]. In brief, 48 h later, MTT was dissolved in phosphate buffered saline (PBS) at a dose of 5 mg/mL and added to all wells (10 μL/well), then the plates were incubated at 37 °C in humidified atmosphere containing 50 mL/L CO2 for an additional 4 h. After incubation, the cells were centrifuged at 1000 r/min for 10 min and all the supernatants were discarded. Then 200 μL of dimethyl sulfoxide was added to each well, and the plates were read 30 sec later in an ELISA plate reader at a wavelength of 490 nm. The absorbance (A) of each well was regarded as the degree of synoviocyte proliferation.
The right legs and hind paws of rats were removed and fixed with 100 g/L paraformaldehyde in PBS, and then decalcified for 10 d with ethylene diamine tetraacetic acid (EDTA) and embedded in paraffin for histologic analysis. The paraffin sections were stained with hematoxylin and eosin. The slides were evaluated histologically by two independent observers, and the gradation of arthritis was scored from 0 to 4 according to the intensity of lining layer hyperplasia, mononuclear cell infiltration, and pannus formation, as described previously[19]: 0, normal ankle joint; 1, normal synovium with occasional mononuclear cells; 2, definite arthritis, a few layers of flat to rounded synovial lining cells and scattered mononuclear cells and dense infiltration with mononuclear cells; 3, clear hyperplasia of the synovium with three or more layers of loosely arranged lining cells and dense infiltration with mononuclear cells; 4, severe synovitis with pannus and erosions of articular cartilages and subchondral bones.
Animals were sacrificed and their small intestines were flushed with 10 mL of sterile saline, followed by separation from bodies. After dried with filter paper, 0.5 g of intestinal specimens was weighed and placed in 5 mL of ice-cold potassium phosphate buffer (pH6.0) with 5 g/L hexadecyl- trimethylammonium bromide. The specimens were homogenized for 20 s (2 × 10 s) and sonicated for 30 s (3 × 10 s) and centrifuged at 12000 g for 15 min at 4 °C. The supernatant was spectrophotometrically assayed for MPO activity by measuring the change in absorbance (at 460 nm) over time. The assay buffer consisted of 50 mmoL/L potassium phosphate, pH6.0 (50 mL), 0.83 mL of H2O2 (3 mL/L solution), and 8.34 mg of O-dianisidine hydrochloride. The supernatant was mixed at 1:80 (supernatant: assay buffer). MPO units were expressed as ΔA/(min·g).
Intestinal DAO activity was determined as follows. In brief, to 0.5 mL homogenate of intestinal specimens gained according to the above method or 0.5 mL dilated standard solution, 3 mL of PBS (0.2 mol/L, pH7.2), 0.1 mL of horseradish peroxidase (4 μg), 0.1 mL of DAO (500 μg), and 0.1 mL of dianisidine were added. After mixed, the mixture was incubated in a water bath at 37 °C for 30 min, then the absorbance value at 436 nm (A436) was recorded after rested for 5 min in the air. The DAO activities were calculated according to the standard curve. Protein activities of the tissue homogenates were determined with Lowry’s method[20]. DAO units were expressed as A/mg pro.
Results were expressed as mean ± SD, and t-test was used to make comparisons between the groups. P < 0.05 was considered statistically significant.
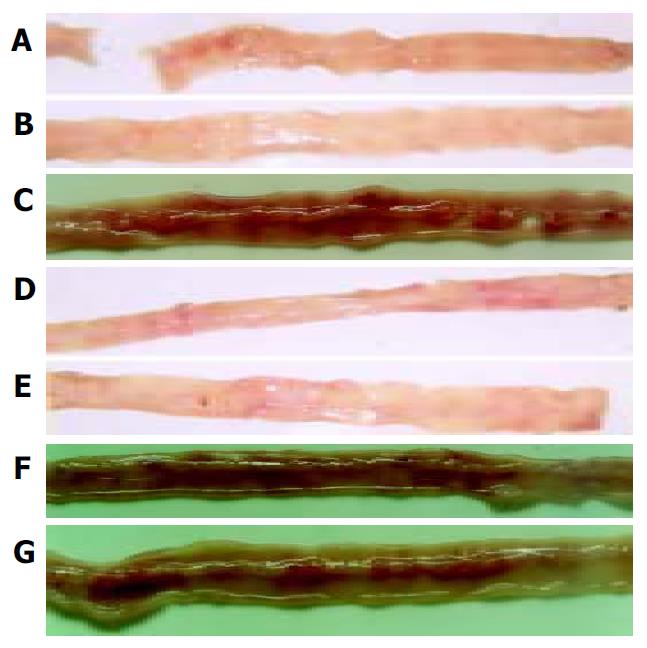
As shown in Figure 1, intragastrical administration of meloxicam (1.5 mg/kg) for 7 d induced multiple intestinal lesions in AA rats. In normal control rats (Figure 1A), AA rats (Figure 1B), AA rats treated with oral CII (Figure 1D) and AA rats treated with nasal CII (Figure 1E), haemorrhage was not observed in antimesenteric side of small intestine (grade 0). In AA rats given meloxicam intragastrically (Figure 1C), AA + meloxicam rats treated with oral CII (Figure 1F) and AA + meloxicam rats treated with nasal CII (Figure 1G), severe haemorrhage was observed in antimesenteric side of small intestine (grade 2). After the animals were treated with oral or nasal administration of CII (20 μg/kg ) for 7 d, development of these lesions was not prevented.
The levels of DAO in intestinal homogenates are shown in Table 1. There was a significant decrease of intestinal DAO activities in AA + meloxicam group (P < 0.01) and AA model group (P < 0.01) compared with normal group. DAO activities of intestinal homogenates in AA + meloxicam group were significantly less than those in AA rats (P < 0.01). Oral or nasal administration of CII had no significant effects on DAO changes.
| Groups | DAO (A/mg pro) | MPO (ΔA/(min·g)) |
| Nomal | 0.37 ± 0.065 | 99.47 ± 22.54 |
| AA model | 0.26 ± 0.031b | 139.80 ± 25.14 |
| AA model + meloxicam | 0.15 ± 0.032ab | 165.42 ± 21.27b |
| AA model + oral CII | 0.27 ± 0.051 | 137.15 ± 26.32 |
| AA model + nasal CII | 0.28 ± 0.029 | 132.17 ± 23.18 |
| AA model + meloxicam + oral CII | 0.15 ± 0.052ab | 159.25 ± 33.36b |
| AA model + meloxicam + nasal CII | 0.16 ± 0.029ab | 152.29 ± 27.20b |
The levels of MPO in intestinal homogenates are shown in Table 1. There was a significant increase of intestinal MPO activities in AA + meloxicam group compared with normal control (P < 0.01). Oral or nasal administration of CII had no significant effects on MPO changes.
As shown in Table 2, secondary arthritis appeared on d 14, and maintained to d 28 after immunization in AA rats (P < 0.01). In AA + meloxicam rats, hind paw secondary swellings were suppressed on d 20 (P < 0.05). The hind paw secondary swellings were suppressed on d 20 in AA model + meloxicam + oral C II group and AA model + meloxicam + nasal C II group (P < 0.05). Oral treatment with CII (20 μg/kg body mass) significantly suppressed hind paw secondary swelling not only on d 24 and 28 in AA rats (P < 0.05), but also on d 24 in AA rats with intestinal lesions induced by meloxicam (P < 0.05). The effects of oral CII were more obvious in AA model than in AA model with intestinal lesions on d 28. While nasal treatment with CII at the same dose significantly suppressed hind paw secondary swelling on d 24 and 28 in both AA rats and AA rats with intestinal lesions (P < 0.01). The effects of nasal CII in AA model were similar to those in AA + meloxicam model, and the effects of nasal CII were more efficient than those of oral CII both in AA model and in AA + meloxicam model (P < 0.05).
| Groups | Dose (μg/kg) | d 14 | d 16 | d 20 | d 24 | d 28 |
| Normal | --- | 0.07 ± 0.05 | 0.12 ± 0.04 | 0.19 ± 0.04 | 0.23 ± 0.03 | 0.26 ± 0.03 |
| AA model | --- | 0.41 ± 0.31d | 0.63 ± 0.16d | 0.99 ± 0.21d | 1.22 ± 0.27d | 1.16 ± 0.21d |
| AA model + meloxicam | --- | 0.29 ± 0.32a | 0.45 ± 0.27a | 0.68 ± 0.18ad | 0.97 ± 0.49d | 0.99 ± 0.32d |
| AA model + oral CII | 20 | 0.39 ± 0.12 | 0.59 ± 0.27 | 0.97 ± 0.15 | 0.81 ± 0.17a | 0.79 ± 0.21a |
| AA model + nasal CII | 20 | 0.38 ± 0.12 | 0.63 ± 0.13 | 1.03 ± 0.10 | 0.69 ± 0.07b | 0.48 ± 0.09bc |
| AA model l + meloxicam + oral CII | 20 | 0.31 ± 0.09 | 0.46 ± 0.26 | 0.70 ± 0.12a | 0.79 ± 0.31a | 0.95 ± 0.25 |
| AA model + meloxicam + nasal CII | 20 | 0.32 ± 0.16 | 0.43 ± 0.19 | 0.73 ± 0.22a | 0.61 ± 0.21b | 0.57 ± 0.17be |
As shown in Table 3, synoviocyte proliferation in AA rats and AA + meloxicam rats was increased (P < 0.01). Oral or nasal administration of CII (20 μg/kg) for 7 d significantly suppressed synoviocyte proliferation (P < 0.01) in AA rats. In AA rats with intestinal lesions, nasal administration of CII (20 μg/kg ) could also significantly suppress synoviocyte proliferation (P < 0.01), while oral administration of CII at the same dosage suppressed synoviocyte proliferation at a lower degree (P < 0.05). There was a significant difference between degrees of synoviocyte proliferation in AA rats with intestinal lesions treated by oral CII and those in the same model treated by nasal CII (P < 0.05).
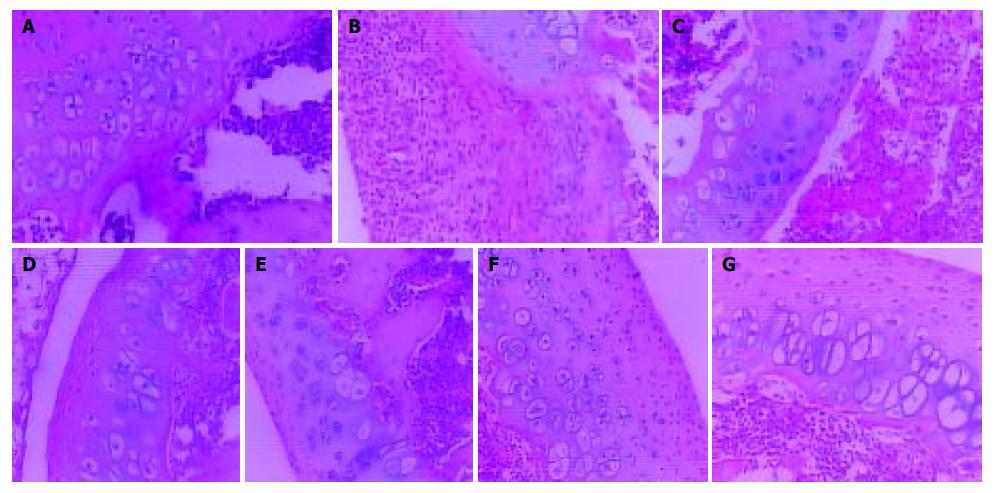
In normal rats, synoviocytes were monolayer (Figure 2A). In AA rats, synoviocytes proliferated three to eight layers and became ovalis types, and articular cartilages were destructed and infiltrated with inflammatory cells. The hyperplastic synovial membranes in AA rats formed a large number of fibroblasts and new blood vessels. Proliferation of collagen fribrils was found under synovial membranes of AA rats (Figure 2B). In AA rats given meloxicam intragastrically, knee joint histopathology was similar to that in AA rats (Figure 2C). In AA rats treated with oral CII, hyperplastic synoviocytes decreased to two or three layers (Figure 2D). In AA rats treated with nasal CII, a few of hyperplastic fiber cells under synovial membranes could be found (Figure 2E). In AA + meloxicam rats treated with oral CII, synovial hyperplasia was observed and articular cartilages were destroyed and infiltrated with inflammatory cells (Figure 2F). In AA + meloxicam rats treated with nasal CII, a few of hyperplastic fiber cells under synovial membranes and partially reversed articular cartilage destrustion could be found (Figure 2G).
Oral or nasal administration of CII could ameliorate the pathologic changes in AA rats or AA + meloxicam rats, but oral administration of CII showed limited efficacy on suppressing the histopathological degradation in AA model with intestinal lesions.
In this study, it was found that intragastric administration of meloxicam (1.5 mg/kg) could induce multiple intestinal lesions in AA rats. The activities of DAO in intestinal homogenates were decreased and the activities of MPO were increased. Oral or nasal administration of CII at a dose of 20 μg/kg could suppress secondary hind paw swelling, synoviocyte proliferation and histopathological degradation in AA model and AA + meloxicam model. Moreover, oral administration of CII showed the limited efficacy on arthritis in AA + meloxicam model and curative effects of nasal CII were shown to be more efficient in comparison with oral CII both in AA model and AA + meloxicam model.
Just like RA, AA is a chronic disease and the ongoing disease can be blocked using antibodies to T cells. They are associated with major histocompatibility complex (MHC) and the primary inflammatory attack is directed to diarthriodial peripheral joints[8].
Thus, development of mucosal tolerance methods for treatment of RA is suitable and could be found successfully both in humans[21,22] and in animal models induced with various heterologous antigens[4-6,23]. Histopathological study showed that oral administration of CII resulted in reduction of synovial hyperplasia, mononuclear infiltration, pannus formation and cartilage erosions[6]. Results from the present study also showed that oral CII suppressed secondary paw swelling, synoviocyte proliferation and histopathological changes in AA rats. Furthermore, clinical studies with oral administration of heterologous CII in RA were carried out and had disputable results[8,21]. Barnett et al[24] found that very low doses tended to give an overall ameliorative effect, whereas higher doses were negative. In addition, intranasal administration of CII could ameliorate ongoing arthritis in pristane-induced arthritis (PIA), collagen-induced arthritis (CIA) and AA[25]. The use of nasal instead of oral administration of the autoantigens has been shown to be more efficient and to require lower tolerogen doses, as demonstrated in several models including CIA in mice[26]. It was also demonstrated that nasal administration of CII in murine CIA model more efficiently inhibited the induction of CIA and CII-specific immune responses than oral adiministration[10]. Our present study indicated that nasal CII could suppress secondary paw swelling, synoviocyte proliferation and histopathological changes in AA rats and was superior to oral CII.
Meloxicam is a NSAID belonging to the enolic acid group of the oxicam family[27]. As a cyclooxygenase - 2 inhibitor, meloxicam has been considered with a good gastric tolerance[28,29]. But it was also reported that meloxicam could induce gastric lesions[30]. Neutrophils, which secrete MPO, appear to be the main effector cells in meloxicam-induced small intestinal damage[31]. On the other hand, there are two major mechanisms which have been put forward to explain the oral tolerance: Active suppression or the antigen specfic induction of regulatory cells and T cell clonal anergy/deletion[32,33]. Cells from PP in GALT were reported to mediate the induction of active suppression[34]. PP cell dysfunction induced by NSAIDs might be the main mechanism of the limited therapeutic effects of oral CII in the suppression of RA in clinical trials. So far, there are few reports to determine it.
In our study, we found that meloxicam (1.5 mg/kg) could induce multiple intestinal lesions in AA rats, and in this model, the activities of DAO were decreased and the activities of MPO were increased. We also found that meloxicam had little effect on AA rats from d 24. These results also indicated that there was a functional turbulence in intestinal systems of AA rats. Furthermore, we found that the effects of oral CII were more obvious in AA model than in AA model with intestinal lesion induced by meloxicam. However, the effects of nasal CII were not influenced by meloxicam in AA rats with intestinal lesions. The results suggested that nasal CII was more efficient than oral CII both in AA rats and in AA rats with intestinal lesions.
In conclusion, at the dose of 20 μg/kg, oral administration of CII has a limited efficacy on arthritis in AA + meloxicam model and the effects of nasal administration of CII on AA are more efficient than that of oral administration of CII both in AA rats and in AA rats with intestinal lesions induced by meloxicam.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Strand V, Kavanaugh AF. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology (Oxford). 2004;43:iii10-iii16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Blake SM, Swift BA. What next for rheumatoid arthritis therapy. Curr Opin Pharmacol. 2004;4:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Wells HG. Studies on the chemistry of anaphylaxis (III). Ex-periments with isolated proteins, especially those of the hen's egg. J Infect Dis. 1911;9:147–171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Hu Y, Zhao W, Qian X, Zhang L. Effects of oral administration of type II collagen on adjuvant arthritis in rats and its mechanisms. Chin Med J (Engl). 2003;116:284-287. [PubMed] |
| 5. | Yoshino S, Quattrocchi E, Weiner HL. Suppression of antigen-induced arthritis in Lewis rats by oral administration of type II collagen. Arthritis Rheum. 1995;38:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Thompson SJ, Thompson HS, Harper N, Day MJ, Coad AJ, Elson CJ, Staines NA. Prevention of pristane-induced arthritis by the oral administration of type II collagen. Immunology. 1993;79:152-157. [PubMed] |
| 7. | Min SY, Hwang SY, Park KS, Lee JS, Lee KE, Kim KW, Jung YO, Koh HJ, Do JH, Kim H. Induction of IL-10-producing CD4+CD25+ T cells in animal model of collagen-induced arthritis by oral administration of type II collagen. Arthritis Res Ther. 2004;6:R213-R219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Choy EH, Scott DL, Kingsley GH, Thomas S, Murphy AG, Staines N, Panayi GS. Control of rheumatoid arthritis by oral tolerance. Arthritis Rheum. 2001;44:1993-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Myers LK, Higgins GC, Finkel TH, Reed AM, Thompson JW, Walton RC, Hendrickson J, Kerr NC, Pandya-Lipman RK, Shlopov BV. Juvenile arthritis and autoimmunity to type II collagen. Arthritis Rheum. 2001;44:1775-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Higuchi K, Kweon MN, Fujihashi K, McGhee JR, Kiyono H. Comparison of nasal and oral tolerance for the prevention of collagen induced murine arthritis. J Rheumatol. 2000;27:1038-1044. [PubMed] |
| 11. | Postlethwaite AE. Can we induce tolerance in rheumatoid arthritis. Curr Rheumatol Rep. 2001;3:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Ding CH, Li Q, Xiong ZY, Zhou AW, Jones G, Xu SY. Oral administration of type II collagen suppresses pro-inflammatory mediator production by synoviocytes in rats with adjuvant arthritis. Clin Exp Immunol. 2003;132:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Combe B, Flipo RM. [What treatments can reduce the digestive complications of NSAIDs]. Presse Med. 2003;32:S33-S37. [PubMed] |
| 14. | Hawkey CJ, Wilson I, Naesdal J, Långström G, Swannell AJ, Yeomans ND. Influence of sex and Helicobacter pylori on development and healing of gastroduodenal lesions in non-steroidal anti-inflammatory drug users. Gut. 2002;51:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Jacobson PB, Morgan SJ, Wilcox DM, Nguyen P, Ratajczak CA, Carlson RP, Harris RR, Nuss M. A new spin on an old model: in vivo evaluation of disease progression by magnetic resonance imaging with respect to standard inflammatory parameters and histopathology in the adjuvant arthritic rat. Arthritis Rheum. 1999;42:2060-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Takeuchi K, Tanaka A, Ohno R, Yokota A. Role of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. J Physiol Pharmacol. 2003;54:165-182. [PubMed] |
| 18. | Russell CA, Vindelov LL. Optimization and comparison of the MTT assay and the 3H-TdR assay for the detection of IL-2 in helper T cell precursor assays. J Immunol Methods. 1998;217:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Chen Q, Wei W. Effects and mechanisms of glucosides of chaenomeles speciosa on collagen-induced arthritis in rats. Int Immunopharmacol. 2003;3:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Tomazic-Jezic VJ, Truscott W. Identification of antigenic and allergenic natural rubber latex proteins by immunoblotting. J Immunoassay Immunochem. 2002;23:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Mimran A, Mor F, Carmi P, Quintana FJ, Rotter V, Cohen IR. DNA vaccination with CD25 protects rats from adjuvant arthritis and induces an antiergotypic response. J Clin Invest. 2004;113:924-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Cazzola M, Antivalle M, Sarzi-Puttini P, Dell'Acqua D, Panni B, Caruso I. Oral type II collagen in the treatment of rheumatoid arthritis. A six-month double blind placebo-controlled study. Clin Exp Rheumatol. 2000;18:571-577. [PubMed] |
| 23. | Bárdos T, Czipri M, Vermes C, Zhang J, Mikecz K, Glant TT. Continuous nasal administration of antigen is critical to maintain tolerance in adoptively transferred autoimmune arthritis in SCID mice. Clin Exp Immunol. 2002;129:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290-297. [DOI] [Full Text] |
| 25. | Lu S, Holmdahl R. Different therapeutic and bystander effects by intranasal administration of homologous type II and type IX collagens on the collagen-induced arthritis and pristane-induced arthritis in rats. Clin Immunol. 1999;90:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL. Suppression of collagen-induced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999;13:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Albengres E, Urien S, Barre J, Nguyen P, Bree F, Jolliet P, Tillement JP, Tsai RS, Carrupt PA, Testa B. Clinical pharmacology of oxicams: new insights into the mechanisms of their dose-dependent toxicity. Int J Tissue React. 1993;15:125-134. [PubMed] |
| 28. | Cryer B, Dubois A. The advent of highly selective inhibitors of cyclooxygenase--a review. Prostaglandins Other Lipid Mediat. 1998;56:341-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Layton D, Harris S, Shakir S. Reply: Re: Layton et al Compari-son of the incidence rates of selected gastrointestinal events reported for patients prescribed rofecoxib and meloxicam in general practice in England using prescription-event monitor-ing data. Rheumatology. 2004;43:681-682. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 30. | Laporte JR, Ibáñez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Villegas I, Martín MJ, La Casa C, Motilva V, De La Lastra CA. Effects of oxicam inhibitors of cyclooxygenase on oxidative stress generation in rat gastric mucosa. A comparative study. Free Radic Res. 2002;36:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Song F, Whitacre CC. The role of the gut lymphoid tissue in induction of oral tolerance. Curr Opin Investig Drugs. 2001;2:1382-1386. [PubMed] |
| 33. | Wu HY, Weiner HL. Oral tolerance. Immunol Res. 2003;28:265-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Toussirot EA. Oral tolerance in the treatment of rheumatoid arthritis. Curr Drug Targets Inflamm Allergy. 2002;1:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |