Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3118
Revised: April 6, 2004
Accepted: April 13, 2004
Published online: November 1, 2004
AIM: To evaluate the value of multi-phasic CT arterial portography (CTAP) and CT hepatic arteriography (CTHA) in differential diagnosis of liver diseases, and to improve the specificity of CTAP and CTHA for liver cancer detection.
METHODS: From January 1999 to December 2002, multi-phasic CTAP and CTHA were performed in 20 patients with suspected liver disease. CT scanning was begun 25 s, 60 s and 120 s for the early-, late- and delayed-phase CTAP examinations, and 6sec, 40 s and 120 s for the early-, late-and delayed-phase CTHA examinations respectively, after a transcatheter arterial injection of non-ionic contrast material. If a lesion was diagnosed as a liver cancer, transcatheter hepatic arterial chemoembolization (TACE) treatment was performed, and the follow-up CT was performed three or four weeks later.
RESULTS: All eighteen HCCs in 12 cases were shown as nodular enhancement on early-phasic CTHA. The density of the whole tumor decreased rapidly on late and delayed phases, and the edge of 12 tumors (12/18) remained relatively hyperdense compared with the surrounding liver tissue, and demonstrated as rim enhancement. All HCCs were shown as perfusion defect nodules on multi-phasic CTAP. Five tumors (5/18) were shown as rim enhancement on delayed-phasic CTAP. Rim enhancement was shown as 1 to 2-mm-wide irregular, uneven and discontinuous circumferential enhancement at late-, and delayed-phase of CTHA or CTAP. Five pseudolesions and 4 hemoangiomas were found in multi-phasic CTAP and CTHA. No pseudolesions and hemoangiomas were shown as rim enhancement on late- or delayed-phasic CTHA and CTAP.
CONCLUSION: Multi-phasic CTAP and CTHA could help to recognize the false-positive findings in CTAP and CTHA images, and improve the accuracy of CTAP and CTHA of liver cancer detection.
- Citation: Li L, Liu LZ, Xie ZM, Mo YX, Zheng L, Ruan CM, Chen L, Wu PH. Multi-phasic CT arterial portography and CT hepatic arteriography improving the accuracy of liver cancer detection. World J Gastroenterol 2004; 10(21): 3118-3121
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3118
CT arterial portography (CTAP) and CT hepatic arteriography (CTHA) are considered as the most sensitive imaging methods of detecting small liver cancers[1,2]. Micro liver cancer as small as 0.2 cm in diameter could be detected by CTAP and CTHA[3]. However, the prevalence of tumor-mimicking benign perfusion abnormalities decreases the specificity of these examinations and the accuracy of tumor detection[4,5]. In order to improve the specificity of CTAP and CTHA for liver cancer detection, multi-phasic helical CT arterial portography (CTAP) and multi-phasic helical CT hepatic arteriography (CTHA) are recommended[6-8]. From January 1999 to December 2002, multi-phasic CTAP and multi-phasic CTHA examinations were performed for more accurate evaluation in 20 patients with suspected liver malignancy.
From January 1999 to December 2002, 20 patients (10 men, 5 women) with suspected liver diseases underwent multi-phasic CTAP and CTHA including 12 cases of hepatocellular carcinomas (HCCs), 3 of cavernous hemangiomas, and 5 of pseudolesions. They aged from 25 to 64 years (mean age, 42.5 years). The serum level of α -fetoprotein (AFP) was elevated in all patients with small HCC, ranged from 410 ng/mL to 2 540 ng/mL. Cirrhosis occurred in all 12 cases with HCC. Five patients with HCC were histologically proven by needle biopsy, and 7 were clinically diagnosed when tumors were demonstrated as lipiodol deposit foci on follow-up CT (lipiodol-CT, Lp-CT) images after transcatheter hepatic arterial chemoembolization (TACE), and the elevated AFP level returned to normal in all patients with HCC.
In this work, we decided a lesion was a pseudolesion when it was proved as normal hepatic tissue by needle liver biopsy, and did not change for at least 1 year. The diagnosis of a cavernous hemangioma was basic on typical findings on dynamic enhanced CT and MRI (very long T2) images, and when it did not enlarge for at least 1 year.
Multi-phasic CTAP examinations were performed with incremental scanning of liver in cranial-to-caudal direction with 2.7-mm to 5-mm collimation on an CT Twin Flash scanner (Philips Corp.). Data acquisition was started 25 s, 60 s and 120 s for the early-, late- and delayed-phase CTAP respectively, after a transcatheter superior mesenteric arterial injection of 40 mL of non-ionic contrast material at 3.0 mL/s, using an automatic power injector (Medrad, Pittsburgh). During the catheterization, contrast material administered before CT scanning was limited to 10 mL injected by hand to visualize aberrant vessels and to facilitate proper catheter placement.
Multi-phasic CTHA examinations were done 20 min after CTAP. Data acquisition was started 6 s, 40 s and 120 s for the early-, late- and delayed-phase CTHA respectively, after the initiation of a transcatheter common hepatic arterial injection of 15 mL of contrast material at 3.0 mL/s.
If serum AFP level exceeded 400 ng/mL, and a lesion was highly suspected as an HCC on CTAP and CTHA images, TACE treatment was recommended to this patient, and plain CT scanning was performed 3 or 4 wk later (Lipiodol CT, Lp-CT).
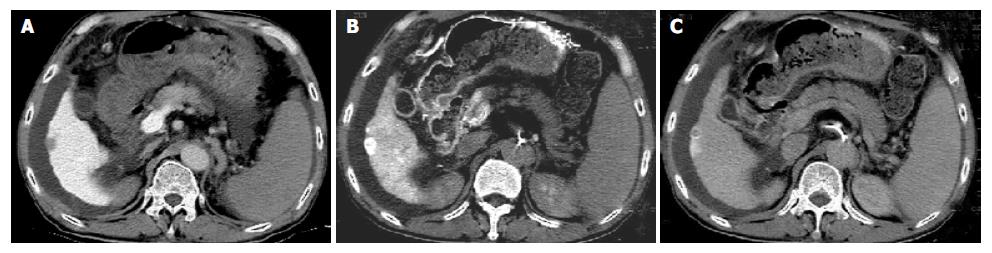
The images of multi-phasic CTAP and CTHA in 20 cases were carefully interpreted by two experienced radiologist. On early-phasic CTHA, all eighteen HCCs were shown as nodular enhancement. The density of the whole tumor decreased rapidly and became hypodense or isodense compared with the surrounding liver tissue on late and delayed phases. However, the edge of tumors (12/18) remained relatively hyperdensity compared with the surrounding liver tissue, and demonstrated as rim enhancement. Rim enhancement was demonstrated as distinct 1 to 2-mm-wide circumferential enhancement at late-, and delayed-phase of multi-phasic CTHA (Figure 1). The shape of enhancement might be irregular, uneven and discontinuous.
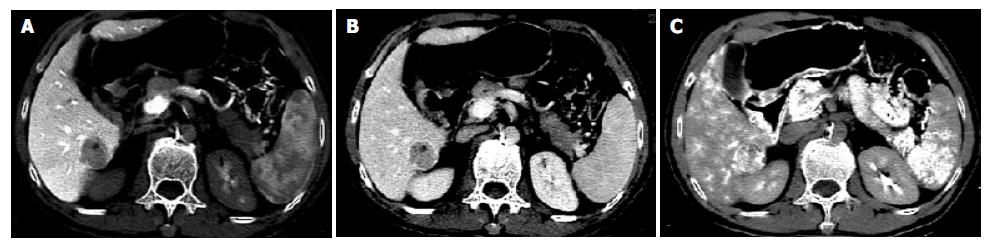
All HCCs were shown as perfusion defect nodules on multi-phasic CTAP. The edge of five HCCs (5/18) became relatively hyperdense compared with the surrounding liver tissue and demonstrated as rim enhancement during delayed CT scanning (Figure 2).
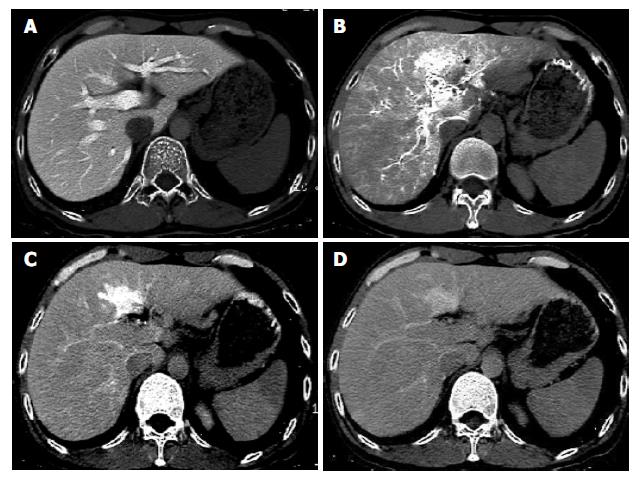
Four of 5 pseudolesions were found in the segment IV of left lobe, and one in the segment VI of right lobe. All pseudolesions were shown as isodense or hypodense compared with the surrounding liver tissue on CTAP images. On CTHA images, pseudolesions were demonstrated as oval, wedge or irregular enhancement. The shape and size of pseudolesions were changed during delayed CT scanning. All pseudolesions gradually became isodense compared with the surrounding liver tissue on delayed phase. No pseudolesions were demonstrated as rim enhancement on late- or delayed-phasic CTHA and CTAP (Figure 3).
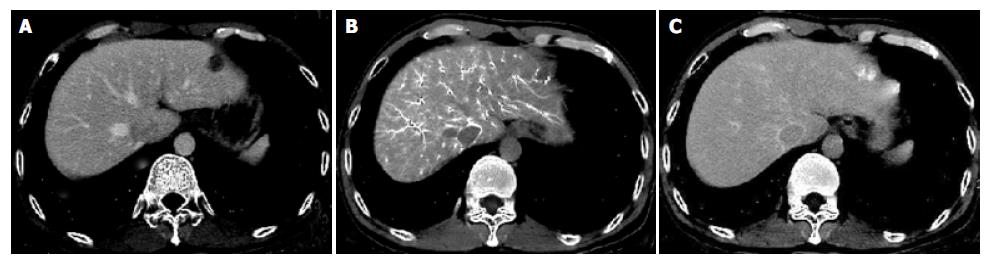
Four hemangiomas of 3 cases were shown as well-defined nodular perfusion defects on multi-phasic CTAP images. On early-phasic CTHA, hemangiomas were demonstrated as peripheral enhancement or nodular enhancement foci. On delayed CT scanning, all hemangiomas became isodense compared with the surrounding liver tissue. There was no hemangioma demonstrated as rim enhancement on late- or delayed-phasic CTHA and CTAP images in our study (Figure 4).
CTAP and CTHA have a high sensitivity for detection of liver diseases[3,9]. Especially, the use of helical CT technique has greatly improved the quality of images and accelerated the scanning[1]. However, the false-positive findings on CTAP and CTHA images have greatly limited its further clinical application[5,10]. Many methods were performed to improve the specificities of CTAP and CTHA for the detection of liver cancer, and combined CTHA and CTHA was recommended to interpret a malignant lesion[9]. Inoue et al[8] and Matsuo et al[6] performed combined CTAP and biphasic CTHA in the pre-operative detection of liver neoplasms. Their results suggested that late-phasic CTHA was useful in differentiating a malignant hepatic tumor from a pseudolesion. Ueda et al[7,11] performed single-level dynamic CTHA in differentiating hypervascular pseudolesion from hypervascular HCC. They found that the stain of pseudolesion on CTHA was transient and could be divided into three phases: (1) inflow of contrast material into the portal vein within the lesion, (2) lesion staining, and (3) fading out of the staining. However, the staining of HCC might have four phases: (1) inflow of contrast material into tumor, (2) tumor staining, (3) inflow of contrast material into adjacent liver, and (4) “coronal staining” of adjacent liver.
Rim enhancement was demonstrated as distinct 1 to 2-mm-wide irregular coronal or circumferential enhancement at late-, and delayed-phase of multi-phasic CTHA or CTAP[12,13]. According to our experience, the indistinct circumferential enhancement was only transiently demonstrated during multi-phase of CTHA and CTAP. Delayed CT scanning 120 s after a transcatheter arterial injection of material might increase the detection of rim enhancement of liver malignancy.
Inoue et al[8] reported that 84% of hepatocellular carcinomas and 73% of hepatic metastases showed rim enhancement on late-phase CTHA, and all non-tumourous perfusions on CTHA images did not show rim enhancement. They concluded that biphasic CTHA was useful in differentiating hepatic malignancy from benign perfusion abnormalities. Similar findings were confirmed by Matsuo et al[6] and Ueda et al[7].
The reason why the malignant tumors showed delayed rim enhancement and pseudolesions did not at late-, or delayed-phase CTHA or CTAP is unclear[14,13]. Ueda et al[7] suggested that rim enhancement in hepatocellular carcinoma was caused by inflow of contrast material from tumor into the adjacent liver. They studied the haemodynamics of hepatocellular carcinoma using single level dynamic CTHA, and demonstrated that arterial blood flowed into the tumor via the hepatic artery, filled the inner part of the tumor, penetrated the capsule, ran through the surrounding hepatic parenchyma and entered the portal branches. The haemodynamics can explain the change of homogeneous enhancement pattern on early-phasic CTHA to rim enhancement on late- or delayed-phasic CTHA. However, the contrast material inside pseudolesions faded out after lesion staining, just like liver tissue.
In our study, rim enhancement usually demonstrated on late-phasic CTHA images could be shown on late- and delayed-phase of CTAP in some cases. The reason of similar findings on CTAP might be that some HCCs could obtain blood supply from portal vein branches surrounding the tumor, especially, in the edge of tumor. This “double” blood supply usually occurred in liver tumors with less vascularity[15]. The border of tumor could be enhanced as a rim of relatively high density during late- and delayed-phase CTAP.
There is a controversy that rim enhancement originates from the normal hepatic tissue surrounding the tumor or from the border of tumor (such as capsule or pseudo-capsule of tumor). Irie et al[12] had performed an CT-pathologic study of metastatic liver tumors, and suggested that rim enhancement of tumor on delayed-phasic CTAP or CTHA images was perilesional enhancement, and it could mainly be caused by an increased supply of arterial flow, a draining of tumorous blood, or an increased interstitial space in the hepatic parenchyma due to fibrotic and desmoplastic changes. However, Semelka et al[14] suggested that rim enhancement might be the enhancement of histological tumor border that was defined as the histologically altered liver parenchyma surrounding the tumor, rather than the outer portion of the tumor.
To our knowledge, it is difficult to distinguish tumor border from perilesional area on CT images. During growth, the tumor infiltrates and compresses surrounding hepatic tissue. The capsule or false-capsule of tumor is formed and contains rich blood flow. It might be difficult to distinguish perilesional enhancement of normal tissue from circumferential enhancement of tumor border on CT and MR images. Rim enhancement demonstrated on late- or delayed-phasic CTHA and CTAP might not be confined to the tumor border because it could extend into the surrounding normal liver tissue.
One of the commonest pseudolesions on CTAP and CTHA images was seen in the liver adjacent to gallbladder or in dorsum of segment IV[4,16]. In our study, four of 5 pseudolesions were found in the left lobe, and one in segment VI of the right lobe. All five pseudolesions demonstrated as isodense lesions or perfusion defects on CTAP images. The shape of enhancement on early-phasic CTHA images might be oval, wedge or irregular. Pseudolesions were changed in shape and size on multi-phasic CTHA images, and gradually became isodense to normal on delayed phase. No pseudolesions were demonstrated as rim enhancement on late- or delayed-phasic CTHA.
Yamagami et al[4] investigated the haemodynamics of pseudolesions using a biphasic CTHA examination, and suggested that pseudolesions on segment IV were caused by direct blood inflow from cholecystic vein to the liver parenchyma, and concluded that later phase CTHA could clearly differentiate pseudolesions from tumors.
Multi-phasic CTAP and CTHA were valuable for differentiating benign lesions from malignancy[10,17]. Hemangiomas were found on multi-phasic CTAP and CTHA images. Four hemangiomas of 3 cases were shown as well-defined perfusion defect nodules on CTAP images, and as nodular enhancement on early-phasic CTHA. On delayed CT scanning, all hemangiomas became isodense to the normal. No hemangioma was demonstrated as rim enhancement on late- or delayed-phasic CTHA images in our study. According to our experience, rim enhancement on multi-phasic CTHA could also be used as a characteristic finding to differentiate malignancy from benign lesions, such as hemangiomas.
In conclusion, multi-phasic CTAP and CTHA could raise the specificity for malignant hepatic tumor detection. Rim enhancement demonstrated on late- and delayed-phasic CTAP and CTHA images might help to differentiate a HCC from benign lesions.
Edited by Zhu LH and Wang XL Proofread by Xu FM
| 1. | Murakami T, Oi H, Hori M, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Hori M, Murakami T, Kim T, Takahashi S, Oi H, Tomoda K, Narumi Y, Nakamura H. Sensitivity of double-phase helical CT during arterial portography for detection of hypervascular hepatocellular carcinoma. J Comput Assist Tomogr. 1998;22:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Li L, Wu PH, Mo YX, Lin HG, Zheng L, Li JQ, Lu LX, Ruan CM, Chen L. CT arterial portography and CT hepatic arteriography in detection of micro liver cancer. World J Gastroenterol. 1999;5:225-227. [PubMed] |
| 4. | Yamagami T, Nakamura T, Kin Y, Nishimura T. Non-tumorous enhancement caused by cholecystic venous inflow shown on biphasic CT hepatic arteriography: comparison with hepatocellular carcinoma. Br J Radiol. 2000;73:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Li L, Wu PH, Lin HG, Li JQ, Mo YX, Zheng L, Lu LX, Ruan CM, Chen L. Findings of non-pathologic perfusion defects by CT arterial portography and non-pathologic enhancement of CT hepatic arteriography. World J Gastroenterol. 1998;4:513-515. [PubMed] |
| 6. | Matsuo M, Kanematsu M, Inaba Y, Matsueda K, Yamagami T, Kondo H, Arai Y, Hoshi H. Pre-operative detection of malignant hepatic tumours: value of combined helical CT during arterial portography and biphasic CT during hepatic arteriography. Clin Radiol. 2001;56:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Ueda K, Matsui O, Kawamori Y, Nakanuma Y, Kadoya M, Yoshikawa J, Gabata T, Nonomura A, Takashima T. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology. 1998;206:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Inoue E, Fujita M, Hosomi N, Sawai Y, Hashimoto T, Kuroda C, Nakano H, Sasaki Y, Ishiguro S. Double phase CT arteriography of the whole liver in the evaluation of hepatic tumors. J Comput Assist Tomogr. 1998;22:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Kim HC, Kim TK, Sung KB, Yoon HK, Kim PN, Ha HK, Kim AY, Kim HJ, Lee MG. Preoperative evaluation of hepatocellular carcinoma: combined use of CT with arterial portography and hepatic arteriography. AJR Am J Roentgenol. 2003;180:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Lim JH, Kim EY, Lee WJ, Lim HK, Do YS, Choo IW, Park CK. Regenerative nodules in liver cirrhosis: findings at CT during arterial portography and CT hepatic arteriography with histopathologic correlation. Radiology. 1999;210:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Ueda K, Matsui O, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Nonomura A, Takashima T. Differentiation of hypervascular hepatic pseudolesions from hepatocellular carcinoma: value of single-level dynamic CT during hepatic arteriography. J Comput Assist Tomogr. 1998;22:703-708. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Irie T, Tsushima Y, Terahata S, Hatsuse K, Kusano S. Rim enhancement in colorectal metastases at CT during infusion hepatic arteriography. Does it represent liver parenchyma or live tumor cell zone. Acta Radiol. 1997;38:416-421. [PubMed] |
| 13. | Terayama N, Matsui O, Ueda K, Kobayashi S, Sanada J, Gabata T, Kawamori Y, Kadoya M. Peritumoral rim enhancement of liver metastasis: hemodynamics observed on single-level dynamic CT during hepatic arteriography and histopathologic correlation. J Comput Assist Tomogr. 2002;26:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Semelka RC, Hussain SM, Marcos HB, Woosley JT. Perilesional enhancement of hepatic metastases: correlation between MR imaging and histopathologic findings-initial observations. Radiology. 2000;215:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Yamagami T, Takeuchi Y, Inaba Y, Matsueda K, Arai Y, Maeda T. Correlation of a defect of portal perfusion in the dorsal part of segment IV of the liver on CT arterial portography with inflow of the aberrant pancreaticoduodenal vein. Br J Radiol. 1999;72:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Onaya H, Itai Y, Satake M, Luo T, Saida Y, Haruno M, Hasebe T, Moriyama N. Highly enhanced hepatic masses seen on CT during arterial portography: early hepatocellular carcinoma and adenomatous hyperplasia. Jpn J Clin Oncol. 2000;30:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |