Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.3044
Revised: January 18, 2004
Accepted: February 12, 2004
Published online: October 15, 2004
AIM: To investigate the expression of three types of mucin (MUC1, MUC2, MUC5AC) and E-cadherin in human gastric carcinomas and their clinical significance.
METHODS: Ninety-four gastric cancer specimens were classified according to WHO criteria and detected by immun-ohistochemical assay of expression of mucins and E-cadherin.
RESULTS: The positive expression rates of MUC1, MUC2, MUC5AC and E-cadherin were 82% (77/94), 84% (79/94), 40% (38/94) and 56% (53/94) respectively. MUC1 expression was significantly correlated with the types of cancer (the positive rates of MUC1 in well and moderately differentiated tubular adenocarcinoma, poorly differentiated adenocarcinoma, signet-ring cell carcinoma and mucinous carcinoma were 91%, 87%, 71%, 71%, respectively, P < 0.05), age of patients (the positive rates of it among the people who are younger than 40 years, between 40-60 years and over 60 year were 74%, 81%, 89%, P < 0.05), lymph nodes involvement (the positive rates in the non-interfered group and the interfered group were 78%, 85%, P < 0.05) and tumor size (the positive rates in the tumors with the size less than 3 cm, 3-6 cm and larger than 6 cm were 69%, 92%, 69%, P < 0.05); MUC2 expression was significantly associated with types of cancers and had the strongest expression in mucinous carcinomas (the positive rates of MUC2 in well and moderately differentiated tubular adenocarcinoma, poorly differentiated adenocarcinoma, signet-ring cell carcinoma and mucinous carcinoma were 94%, 70%, 81%, 100%, P < 0.05), but it had no obvious relation to age, gender, tumor location, lymph nodes involvement, depth of invasion and metastasis to extra-gastric organs (P > 0.05); MUC5AC expression was not related to any of the characteristics investigated except that it had relation to gender, whereas MUC5AC showed the tendency to higher expression in less invasive lesions and lower expression in advanced stage cancers (P > 0.05); No significant difference was found for E-cadherin expression. There were strong positive relationships between the expression of MUC1 and E-cadherin, MUC2 and E-cadherin, MUC1 and MUC2 (R = 0.33, R = 0.22, R = 0.32, respectively, P < 0.05). According to the COX proportional hazards model, older patients, involvement of lymph nodes, different types of gastric cancer and MUC2 expression were significantly associated with poorer outcome of gastric carcinoma patients (β = 0.08, β = 3.94, β = 1.33, β = 0.75, respectively, P < 0.05).
CONCLUSION: MUC1 and MUC2 are good markers of different types of gastric cancer. MUC2 is especially a good marker of mucinous carcinoma. MUC1, MUC2 may interfere with the function of E-cadherin in gastric carcinomas, and have synergic effect on progression of gastric cancers.
- Citation: Zhang HK, Zhang QM, Zhao TH, Li YY, Yi YF. Expression of mucins and E-cadherin in gastric carcinoma and their clinical significance. World J Gastroenterol 2004; 10(20): 3044-3047
- URL: https://www.wjgnet.com/1007-9327/full/v10/i20/3044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.3044
Mucins, the high molecular weight glycoproteins that contain oligosaccharides, are the major components of the mucous gel covering the surface of epithelial tissue. Their main functions are thought to be lubrication and protection of the epithelial surface[1,2]. To date, at least thirteen mucins have been found[3].
MUC1, an epithelial mucin glycoprotein, is highly expressed in lactating mammary glands[4]. Under pathological conditions, such as colon adnenocarcinoma and pancreas adnenocarcinoma or stomach adnenocarcinoma, MUC1 would change its expression fashion and rate[5]. MUC2, a gel-forming mucin, highly expresses in normal intestinal tissues and has no expression in normal gastric mucosa. However, it had de novo expression in stomach when the mucosa underwent metaplasia and carcinoma[6]. MUC5AC, a gastric type mucin in gastric cardia and body mucosa, is decreased when the tissue has cancers[7]. E-cadherin is a calcium-dependent cell-cell adhesion molecule and its expression decreases in the carcinoma tissues thus contributing to cancer progression and correlate with patients’ prognosis[8].
Despite many studies have been done in gastric carcinoma tissue, the results are still in contradiction. Some reported that MUC1 could well predict the patients’ prognosis, while others thought not. MUC2 showed the same result[9,10]. MUC5AC is a relatively less studied molecule, and its expression decreases in advanced cancers than in early cancers[11]. Study on E-cadherin also remains contradictory[12,13]. What’s more, few studies have been done about their relationships, especially between E-cadherin and the mucins. The present study was designed to provide some useful information on these molecules.
Ninety-four patients with gastric adenocarcinomas confirmed pathologically and underwent gastrectomy in our hospital from January 1989 to December 2000 were Systemically selected for the study. Patients’ age, gender, tumor location, depth of invasion, local lymph nodes involvement, metastasis, tumor size were all obtained from the original records. Specimens were histologically classified according to WHO criteria by two experienced pathologists . Among these subjects, there were 33 moderately and highly-differentiated tubular carcinomas, 23 poorly differentiated adenocarcinomas, 31 signet-ring cell carcinomas and 7 mucinous carcinomas. There were 64 male and 30 female patients with a mean age of 52.1 ± 12.1 years (range 25-75 years). The mean tumor size was 4.5 ± 2.0 cm in diameter (range 1-10 cm). A total of 48 patients provided full information during follow-up.
Antibodies against MUC1, MUC2 and MUC5AC were purchased from Shenzhen Jingmei Biotechnology, Inc., and the antibody against E-cadherin was from Fujian Maixin Co, Ltd. All the 94 formalin-fixed, paraffin-embedded specimens were sliced sequentially with a thickness of 4 μm. According to the protocol, these tissue sections were dewaxed, rehydrated, incubated with 30 mL/L hydrogen peroxide in methanol for 30 min to block endogenous peroxidase, and then washed with PBS (phosphate buffered saline, pH 7.4). After that, they were incubated with non-immunized horse serum for 30 min at room temperature, washed again, and incubated with the specific antibodies overnight at 4 °C or 1 h at 37 °C. They were washed again, incubated with the secondary antibody (Biotin labeled goat anti-rabbit antibody) and streptavidin-biotin peroxidase for 30 min separately, visualized with 3,3’-diaminobenzidine tetra-hydrochloride and H2O2, counterstained with haematoxylin. Primary antibodies were replaced with PBS buffer as negative control.
In order to obtain a more precise relation between mucins and the clinical indicators, a semiquantitative analysis was performed to evaluate positively stained cells in carcinoma tissues as + + +, + +, + or -. We examined 10 fields of each cancerous tissue at high magnification ( × 400) and scored the intensity of color as 0 for non-stained,1 for the color of yellow, 2 for brown-yellow, 3 for brown; the rate of positive cell was judged as negative (0) if it was less than 5%, 1 for 5%-25%, 2 for 26%-50%, 3 for over 50%. The mean intensity scores were multiplied by the rate scores. Negative group ( - ) was defined when the result was 0, 1-3 was mild-positive ( + ), 4-5 was moderate-positive ( + + ) and equal or greater than 6 was strong positive ( + + + ).
χ2 test, the hazards proportional analysis (COX) and the correlation analysis were used to determine differences between groups using SPSS 10.0 software. Statistical significance was established at P < 0.05.
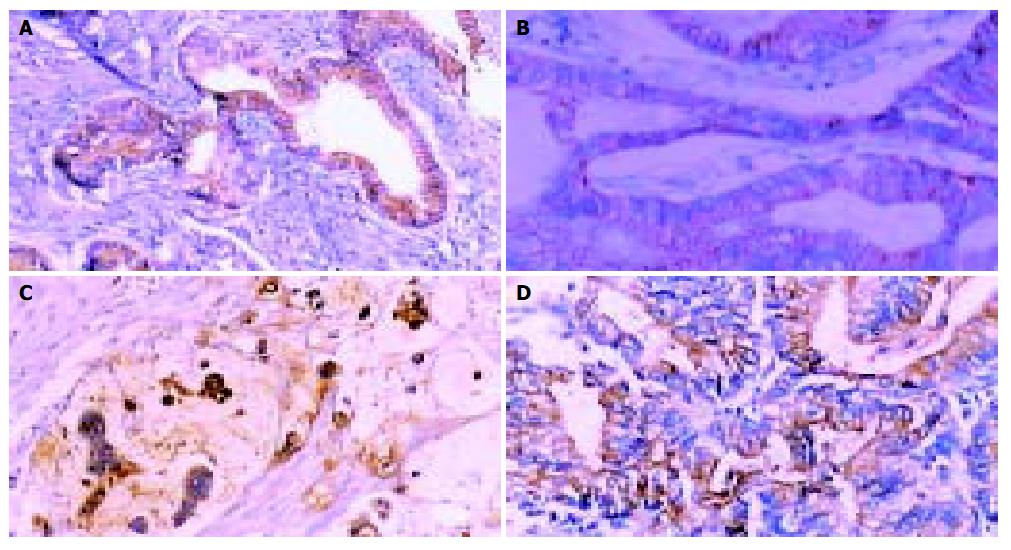
The positivity rate of MUC1 was 82% in the cancerous tissues. It expressed in the cytoplasm diffusely or stained on the membrane of the cells. There were significant differences in its expression among different types of the cancer (P < 0.05) with the highest expression rate in well and moderately differentiated tubular adenocarcinomas (91%). Moreover, its expression level had significant relationship with patients’ age, local lymph nodes involvement and tumor size (P < 0.05). MUC2 had a positive expression rate of 84%, with the highest expression level in mucinous carcinomas (100%) and lowest expression level in signet-ring cell carcinoma (19%). Furthermore, it also showed significant differences in expression among different types of cancer (P < 0.05). MUC5AC had a positive expression rate of 40% and the lowest expression level in mucinous carcinoma (14%), but no significant differences in expression levels among different types of cancer were found (P > 0.05). It expressed mainly in the cytoplasm. E-cadherin had a positive expression rate of 56% with the highest expression in well and moderately differentiated cancers (70%) and the lowest in mucinous type (29%). There was no significant difference of expression level among different types of cancer (P > 0.05). Its positive expression was on the membrane and in the cytoplasm of cancerous cells. The expression of MUC2, MUC5AC, E-cadherin were not significantly different with regards to clinicopathological characteristics (Table 1, Figure 1).
| n | MUC1 | MUC2 | MUC5AC | E-cadherin | |||||||||||||
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | ||
| Types of cancer | P = 0.005 | P = 0.001 | NS | NS | |||||||||||||
| WMDTA | 33 | 3 | 2 | 3 | 25 | 2 | 3 | 7 | 21 | 21 | 6 | 0 | 6 | 10 | 8 | 5 | 10 |
| PDA | 23 | 3 | 11 | 2 | 7 | 7 | 6 | 5 | 5 | 15 | 4 | 1 | 3 | 11 | 5 | 3 | 4 |
| SRCC | 31 | 9 | 10 | 2 | 10 | 6 | 9 | 10 | 6 | 14 | 5 | 8 | 15 | 8 | 4 | 4 | |
| MC | 7 | 2 | 1 | 0 | 4 | 0 | 0 | 0 | 7 | 6 | 0 | 0 | 1 | 5 | 2 | 0 | 0 |
| Age(yr) | P = 0.03 | NS | NS | NS | |||||||||||||
| 4 0 | 19 | 5 | 4 | 2 | 8 | 3 | 6 | 5 | 5 | 12 | 2 | 2 | 3 | 10 | 7 | 1 | 1 |
| > 40, ≤ 60 | 48 | 9 | 18 | 1 | 20 | 10 | 9 | 10 | 19 | 26 | 10 | 3 | 9 | 20 | 9 | 8 | 11 |
| > 60 | 27 | 3 | 2 | 4 | 18 | 3 | 2 | 7 | 15 | 18 | 2 | 1 | 6 | 11 | 7 | 3 | 6 |
| Sex | NS | NS | P = 0.02 | NS | |||||||||||||
| male | 64 | 13 | 14 | 4 | 33 | 10 | 10 | 14 | 30 | 42 | 5 | 3 | 14 | 28 | 17 | 7 | 12 |
| female | 30 | 4 | 10 | 3 | 13 | 6 | 7 | 8 | 9 | 14 | 9 | 3 | 4 | 13 | 6 | 5 | 6 |
| Location | NS | NS | NS | NS | |||||||||||||
| Upper 1/3 | 13 | 0 | 4 | 1 | 8 | 1 | 2 | 0 | 10 | 12 | 0 | 0 | 1 | 3 | 4 | 1 | 5 |
| Middle 1/3 | 23 | 6 | 6 | 0 | 11 | 4 | 3 | 6 | 10 | 12 | 3 | 2 | 6 | 11 | 7 | 2 | 3 |
| Lower 1/3 | 58 | 11 | 14 | 6 | 27 | 11 | 12 | 16 | 19 | 32 | 11 | 4 | 11 | 27 | 12 | 9 | 10 |
| Invasion | NS | NS | NS | NS | |||||||||||||
| within mucosa & sub-mucosa | 7 | 2 | 1 | 0 | 4 | 3 | 2 | 0 | 2 | 5 | 0 | 0 | 2 | 4 | 1 | 0 | 2 |
| Muscular layer | 19 | 5 | 7 | 1 | 6 | 5 | 4 | 6 | 4 | 9 | 1 | 3 | 6 | 9 | 3 | 5 | 2 |
| Serosa | 68 | 10 | 16 | 6 | 36 | 8 | 11 | 16 | 33 | 42 | 13 | 3 | 10 | 28 | 19 | 7 | 14 |
| Metastasis to LN | P = 0.031 | NS | NS | NS | |||||||||||||
| No | 40 | 9 | 14 | 10 | 7 | 9 | 9 | 11 | 11 | 25 | 4 | 3 | 8 | 20 | 8 | 5 | 7 |
| Yes | 54 | 8 | 10 | 7 | 29 | 7 | 8 | 11 | 28 | 31 | 10 | 3 | 10 | 21 | 15 | 7 | 11 |
| Metastasis to other organs | NS | NS | NS | NS | |||||||||||||
| No | 70 | 14 | 19 | 7 | 30 | 13 | 12 | 18 | 27 | 42 | 7 | 5 | 16 | 32 | 19 | 8 | 11 |
| Yes | 24 | 3 | 5 | 0 | 16 | 3 | 5 | 4 | 12 | 14 | 7 | 1 | 2 | 9 | 4 | 4 | 7 |
| Tumor size (cm) | P = 0.044 | NS | NS | NS | |||||||||||||
| ≤ 3 | 29 | 9 | 4 | 4 | 12 | 9 | 6 | 6 | 8 | 17 | 5 | 0 | 7 | 12 | 7 | 3 | 7 |
| 3-6 | 52 | 4 | 17 | 3 | 28 | 5 | 10 | 15 | 22 | 29 | 9 | 5 | 9 | 20 | 15 | 6 | 11 |
| > 6 | 13 | 4 | 3 | 0 | 6 | 2 | 1 | 1 | 9 | 10 | 0 | 1 | 2 | 9 | 1 | 3 | 0 |
There were significantly positive relationships between MUC1 and MUC2, MUC1 and E-cadherin, MUC2 and E-cadherin (P < 0.05). According to COX analysis, patients’ age, lymph node involvement, types of the cancer and the level of MUC2 expression were the factors related to patients’ survival after operation (figures not shown).
We confirmed that mucin expression was associated with differentiation characteristics of gastric carcinoma. MUC1 was expressed in most of the studied gastric cancerous specimens (82%), being consistent with other studies[10]. MUC1 expression related to clinical characteristics such as patients’ age, tumor size and local lymph nodes involvement. MUC1 expressed higher in older patients with larger tumor or with more ymph nodes involvement. On the other hand, though the expression of MUC1 was not significantly associated with the metastasis and depth of invasion (P > 0.05), it still had the tendency toward higher expression in advanced stage of cancer. However, we failed to find that MUC1 had the prognostic role in gastric cancer patients, which is different with Ustsunomiya’s conclusion[9], but consistent with Reis’s conclusion[14]. These contradictory results might be due to our relatively small number of follow-up patients after operation.
MUC2, the intestinal mucin, expressed in most of the studied cases (86%), higher than that in other studies[15], However our result was in accordance with other results concerning its overwhelming expression in mucinous carcinomas[15,16]. Contradictory to some reports that MUC2 indicated good prognosis[9], our study found that MUC2 could predict poor outcome. Our in vitro study used anti-sense oligonucleotide of MUC2 to inhibit the growth of gastric cancer cells, while Sternberg used the anti-sense oligonucleotide of MUC2 in colon cells in vitro and in vivo and found that it decreased the adherence ability of cells to E-selectin and resulted in inhibition of liver metastasis[17]. Both supported our conclusion that MUC2 might contribute to gastric cancer progress.
MUC5AC was thought to be gastric mucin and expressed higher in early stage of cancers than in the advanced stage[18]. MUC5AC rarely expressed in mucinous carcinoma except in one case. Between different sex groups, MUC5AC had significant difference (P < 0.05).
E-cadherin is a calcium-dependent molecule, and acts as a tumor-inhibitory factor. Some studies have shown that the lower expression level it had, the faster the tumor progressed[12]. But we could not draw a conclusion like this despite the tendency shown at present. While we noted its highest expression in the well and moderately differentiated tubular carcinoma which supported the view on its contribution to tubular structure formation[19].
Although some studies showed that MUC1 expression in gastric cancers was negatively correlated with the expression of E-cadherin[20], we found the positive relationships between them, what’s more, the correlation of MUC1, MUC2 and E-cadherin were first studied by us. Both MUC1 and MUC2 might contribute to the progress of gastric cancer, and they might restrain the role of E-cadherin.
In summary, MUC1 may contribute to gastric cancers progress, larger tumor size and metastasis to lymph nodes, at the same time, it may inhibit E-cadherin. MUC2 had the same role as MUC1,besides, it may be an indicator for prognosis of gastric cancer patients and good marker for mucinous cancers. E-cadherin could not be used a tangible marker to indicate gastric cancers progress but may play a role in the tubular formation. The role of MUC5AC in gastric cancers needs more investigation.
The authors express their gratitude to Dr. Jie Chen and Dr. Fu-Sheng Liu for their precious advice and suggestions. We thank Mr. Da-Hai Sun for his assistance in preparation of the manuscript.
Edited by Chen WW and Zhu LH Proofread by Xu FM
| 1. | Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192-D1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327-18336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286-15293. [PubMed] |
| 5. | Seregni E, Botti C, Massaron S, Lombardo C, Capobianco A, Bogni A, Bombardieri E. Structure, function and gene expression of epithelial mucins. Tumori. 1997;83:625-632. [PubMed] |
| 6. | Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681-2690. [PubMed] |
| 7. | Guyonnet Duperat V, Audie JP, Debailleul V, Laine A, Buisine MP, Galiegue-Zouitina S, Pigny P, Degand P, Aubert JP, Porchet N. Characterization of the human mucin gene MUC5AC: a consensus cysteine-rich domain for 11p15 mucin genes? Biochem J. 1995;305:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Berx G, Staes K, van Hengel J, Molemans F, Bussemakers MJ, van Bokhoven A, van Roy F. Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1). Genomics. 1995;26:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998;4:2605-2614. [PubMed] |
| 10. | Baldus SE, Zirbes TK, Engel S, Hanisch FG, Mönig SP, Lorenzen J, Glossmann J, Fromm S, Thiele J, Pichlmaier H. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int J Cancer. 1998;79:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Reis CA, David L, Carvalho F, Mandel U, de Bolós C, Mirgorodskaya E, Clausen H, Sobrinho-Simões M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, Hommel G. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996;69:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Blok P, Craanen ME, Dekker W, Tytgat GN. Loss of E-cadherin expression in early gastric cancer. Histopathology. 1999;34:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Reis CA, David L, Seixas M, Burchell J, Sobrinho-Simões M. Expression of fully and under-glycosylated forms of MUC1 mucin in gastric carcinoma. Int J Cancer. 1998;79:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Pinto-de-Sousa J, David L, Reis CA, Gomes R, Silva L, Pimenta A. Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch. 2002;440:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hanski C, Hofmeier M, Schmitt-Gräff A, Riede E, Hanski ML, Borchard F, Sieber E, Niedobitek F, Foss HD, Stein H. Overexpression or ectopic expression of MUC2 is the common property of mucinous carcinomas of the colon, pancreas, breast, and ovary. J Pathol. 1997;182:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Sternberg LR, Byrd JC, Yunker CK, Dudas S, Hoon VK, Bresalier RS. Liver colonization by human colon cancer cells is reduced by antisense inhibition of MUC2 mucin synthesis. Gastroenterology. 1999;116:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s-1943s. [PubMed] |
| 20. | Tanaka M, Kitajima Y, Sato S, Miyazaki K. Combined evaluation of mucin antigen and E-cadherin expression may help select patients with gastric cancer suitable for minimally invasive therapy. Br J Surg. 2003;90:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |