Published online Jan 15, 2004. doi: 10.3748/wjg.v10.i2.195
Revised: August 8, 2003
Accepted: August 16, 2003
Published online: January 15, 2004
AIM: To investigate the expression of 4-1BB molecule in hepatocellular carcinoma (HCC) and its adjacent tissues.
METHODS: Reverse transcription–polymerase chain reaction (RT-PCR) was used to determine the gene expression of 4-1BB in hepatocarcinoma and its adjacent tissues, and peripheral blood mononuclear cells (PBMCs) from both HCC and health control groups. Flow cytometry was used to analyse the phenotypes of T cell subsets from the blood of HCC patients and healthy volunteers, and further to determine whether 4-1BB molecules were also expressed on the surface of CD4+ and CD8+ T cells. The localization of 4-1BB proteins on tumor infiltrating T cells was determined by direct immunofluorescence cytochemical staining and detected by confocal microscopy.
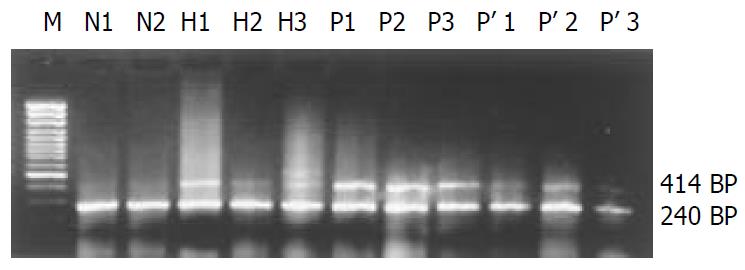
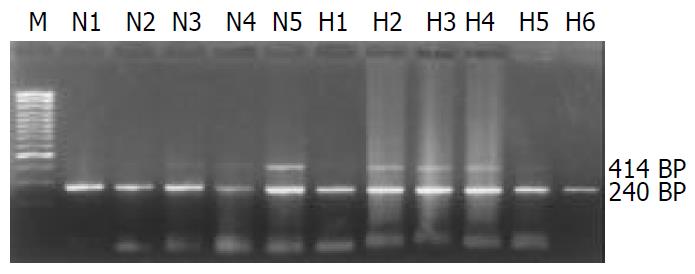
RESULTS: 4-1BB mRNA, which was not detectable in normal liver, was found in 19 liver tissues adjacent to tumor edge (< 1.0 cm). Low expression of 4-1BB mRNA was shown in 8 tumor tissues and 6 liver tissues located within 1 to 5 cm away from tumor edge. In PBMCs, 4-1BB mRNA was almost not detected. Percentage of CD4+, CD8+ and CD3+/CD25+ T cells, as well as ratio of CD4 to CD8 revealed no difference between groups (P > 0.05, respectively), while a significant lower percentage of CD3+ T cell was found in HCC group as compared to healthy control group (P < 0.05). However, 4-1BB molecules were almost not found on the surface of CD4+ and CD8+ T cells in HCC and healthy control group. Double-staining of 4-1BB+/CD4+ and 4-1BB+/CD8+ immunofluorescence on tumor infiltrating T cells was detected in 13 liver tissues adjacent to tumor edge (< 1.0 cm) by confocal microscopy.
CONCLUSION: Although HCC may escape from immune attack by weak immunogenicity or downregulated expression of MHC-1 molecules on the tumor cell surface, tumor infiltrating T cells can be activated via other costimulatory signal pathways to exert a limited antitumor effect on local microenvironment. The present study also implicates that modulating 4-1BB/4-1BBL costimulatory pathway may be an effective immunotherapy strateg to augment the host response.
- Citation: Wan YL, Zheng SS, Zhao ZC, Li MW, Jia CK, Zhang H. Expression of co-stimulator 4-1BB molecule in hepatocellular carcinoma and adjacent non-tumor liver tissue, and its possible role in tumor immunity. World J Gastroenterol 2004; 10(2): 195-199
- URL: https://www.wjgnet.com/1007-9327/full/v10/i2/195.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i2.195
Hepatocellular carcinoma (HCC) has a very poor prognosis owing to its high malignancy, and it ranks second cause of cancer death in China[1]. Curative tumour resection or orthotopic liver transplantation (LTx) seems to be an optimal treatment. Nevertheless, the recurrence rate remains high both after tumour resection and LTx[2-10]. Chemotherapy and embolisation are at best palliative with few impacts on survival[3]. Recently, immunotherapy has been used with some success for such tumours as melanoma[11-14] and renal-cell carcinoma[15,16] that are associated with an inflammatory or immune response. However, like most solid tumours, HCC has long been considered poorly immunogenic and substantially refractory to immunotherapy. Since a better prognosis of HCC attributes to the anti-tumor effect induced by cellular immunity of infiltrating CD8+ and CD4+ T lymphocytes[17], these tumor infiltrating lymphocytes (TILs) might be in an activated status and play a limited immune protection in microenvironment.
The 4-1BB receptor, a recently identified molecule of tumor necrosis factor- receptor (TNFR) superfamily, is a type I membrane protein expressed on activated cytolytic and helper T cells[18,19], as well as NK cells[20]. The ligand for 4-1BB receptor is a 4-1BB ligand (4-1BBL), which is expressed on APCs including B cells, macrophages, and dendritic cells[21,22]. Ligation of 4-1BB with 4-1BB ligand plays an important role in sustaining T cells activation, amplifying cytotoxic T lymphocyte (CTL) response, as well as inducing IL-2 production in the complete absence of a signal through CD28 molecule[22,23]. A recent research demonstrated that immunomodulatory gene therapy with 4-1BB ligand could induce long-term remission of liver metastases in a mouse model and augment CTL response against tumor[24]. The present study was to detect whether 4-1BB molecules were expressed on infiltrating CD4+ and CD8+ T cells in HCC and its adjacent tissues, and to illustrate the role of 4-1BB/4-1BBL pathway in tumor immunity.
Nineteen patients with HCC confirmed by histopathologic examination were selected. Among them, 14 were male and 5 female aged from 28 to 68 years (average, 49.67 ± 13.04 years). Three liver specimens from mismatched cadaver donor and 22 healthy peripheral blood specimens from Blood Center of Zhejiang Province were served as controls.
Fluorescein isothiocyanate (FITC) -conjugated mouse monoclonal antibodies (mAbs) specific for human surface antigens including anti-CD4 (IgG1k clone RPA-T4), anti-CD8 (IgG1k clone RPA-T8), anti-CD3 (IgG1k clone UCHT1), phycoerythrin (PE)-conjugated anti- CD25 (IgG1k clone M-A251), anti-4-1BB (IgG1k clone 4B4-1), and FITL or PE-conjugated mouse IgG1k (clone MOPC-21) as isotype controls were purchased from Becton Dickinson, San Jose, CA. RevertAidTM M-MuLV reverse transcriptase and Taq DNA polymerase were obtained from Promega, USA.
In order to isolate PBMCs, 5 ml heparinized blood was diluted 1:1 with PBS containing 0.6% Na3-citrate and layered over a 5 ml Ficoll cushion. After centrifugation (20 min, 700×g), the interface containing PBMCs was collected and washed twice with PBS. This precipitate contained approximately 25% monocytes and 75% lymphocytes.
Semi-quantitative assessment of 4-1BB mRNA expression was performed using RT-PCR on a PTC-200 DNA engine (MJ Research, USA). Briefly, total RNA was prepared from PBMCs and liver tissues using TRIZOL (Gibco BRL Life Technologies, Breda, the Netherlands). cDNA was synthesized with 2 μg of total RNA template using the superscript pre-amplification system (Promega, USA) and random primers in a final volume of 20 μl. The cDNA used as template was checked in respect to human β2-MG amplification. The following primers (Shanghai Sangon, China) were used: β2-MG sense primer: 5’-CCAGCAGAGAATGGAAAGTC-3’, β2-MG antisense primer: 5’- GATGCTGCTTACATGTCTCG-3’, 4-1BB sense primer: 5’-TCAGGACCAGGAAGGAGTGT-3’, 4-1BB antisense primer: 5’-AACGGAGCGTGAGGAAGAAC-3’. Using these primers, fragments of 240 bp, and 414 bp were expected to result from amplification of β2-MG and 4-1BB cDNAs, respectively. PCR reactions contained 20 pmol of each primer for 4-1BB, 2.5 u of Taq polymerase, 1 μl of 25 mM dNTPs, 1.2 μl of 25 mM MgCl2 and 10×PCR buffer in a final volume of 25 μl. For β2-MG amplification, 1 μl of each primer at a 1:8 diluted concentration to 4-1BB primers was used for the reaction. PCR products (8 μl) were analyzed on 1.5% agarose gel containing ethidium bromide using Kodak DNA analyser (Gibco BRL) with Kodak digital science 1S 2.0 software. The expression level of 4-1BB mRNA was described as the ratio of 4-1BB/β2-MG×100.
One hundred microliters of heparinized peripheral blood were incubated with monoclonal antibody at room temperature in dark for 15 min to 30 min according to the manufacturer’s instructions. Another 100 μl of heparinized peripheral blood incubated with FITL or PE-conjugated mouse IgG1k (clone MOPC-21) was used as negative isotype control. Erythrocytes were lysed in turn with ImmunoPrep A, B, and C haemolytic solution on Coulter Q-Prep (Beckman-coulter). Alignment was checked using immunocheck beads (Beckman-coulter). All results were obtained using EPICS® XL FACScan (Beckman-coulter) with systemIII software.
Tissues were stored at -70 °C until use. Four μm-thick frozen sections (on poly-L-lysine coated slides) were fixed in acetone for 10 min at 4 °C. The sections were blocked in phosphate buffered saline (PBS) and 1% bovine serum albumin (BSA) for 1 h, followed by incubation with FITC and PE labeled antibodies or conjugated isotype matched control antibodies for 16 h at 4 °C. After extensively washed (overnight), stained sections were covered in PBS and kept in dark at 4 °C.
LEICA TCS-SP confocal microscope (Germany) was equipped with argon lasers and Leica inverted research biological microscope with an oil immersion objective lens of×40 (NA1.30). The sections processed for immunocytochemistry were viewed under LEICA TCS-SP confocal microscope. After standard fluorescence observations, 4-1BB and CD4 or CD8 localization on TIL was automatically scanned by laser emitted at 488 nm and imaged by using PowerScanner physiology software. FITC and PE fluorescence emissions were captured through grating at 530/30-nm and 605/30-nm respectively.
Data were expressed as mean ± SD. Statistical analysis was performed using one-way ANOVA with SPSS 10.0 software. Kruskal-Wallis H test and Student’s t test were also used for the nonparametric and parametric data analysis between two groups, respectively. A P value ≤ 0.05 was considered significant.
4-1BB mRNA was not detectable in normal liver, but was detected in all 19 liver tissues adjacent to tumor edge (< 1.0 cm). Low expression of 4-1BB mRNA was shown in 8 tumor tissues and 6 liver tissues located within 1 to 5 cm away from tumor edge. However, in PBMCs, 4-1BB mRNA expression was not detected in samples from 18 healthy controls (81.82%, 18/22) and 13 patients with HCC (68.42%, 13/19). Very low expression of 4-1BB mRNA was detected in another 4 healthy volunteers and 6 patients with HCC. However, the median level of 4-1BB mRNA expression from PBMCs in each group was 0 (P > 0.05) (Figure 1, Figure 2).
In order to analyze T cell phenotypes, and determine whether 4-1BB molecules expressed on T cells from PBMCs, flow cytometric analysis was used. The results are shown in Table 1. Percentages of CD4+, CD8+ T cells and CD3+CD25+ T cells, as well as the ratio of CD4 to CD8 had no significant differences between the groups (P > 0.05). However, a significantly higher percentage of CD3+ T cells was found in healthy control group as compared to HCC group (P < 0.05). 4-1BB molecules were almost not found on the surface of CD4+ and CD8+ T cells in HCC and healthy control group. The percentage of 4-1BB+/CD4+ or CD8+ T cells in two groups was not more than 0.1%, and the median value in each group was 0 (P = 0.406 for 4-1BB+/CD4+, P = 0.209 for 4-1BB+ /CD8+, respectively). (Table1, Figure 3).
| Groups | n | CD4 | CD8 | CD3 | CD3/CD25 | CD4/CD8 |
| Normal control | 22 | 34.74 ± 6.19 | 28.70 ± 5.11 | 66.56 ± 10.24 | 1.22 ± 0.13 | 1.20 ± 0.18 |
| HCC | 19 | 31.40 ± 4.70 | 25.83 ± 3.98 | 61.30 ± 4.61 | 1.22 ± 0.12 | 1.26 ± 0.17 |
| P value | 0.058 | 0.051 | 0.038 | 0.918 | 0.279 |
To determine whether TILs were the same clones as T lymphocytes from peripheral blood, confocal laser microscopy was used to detect 4-1BB expressions on TILs. As expected, we did not find any PE conjugated 4-1BB and FITC conjugated CD4 or CD8 fluorescence located within 3 normal liver tissues. However, co-localization of 4-1BB+/CD4+ or CD8+ on TIL was visualized by confocal laser microscopy in 3 tumor tissues and 1 liver tissue located within 1 to 5 cm away from tumor edge, as well as 13 tumor adjacent tissues within 1 cm. (Figure 4).
It seems that a better prognosis of HCC could attribute to the anti-tumor effect induced by cellular immunity of infiltrating CD8+ and CD4+ T lymphocytes[17]. However, like most solid tumours, HCC has long been considered poorly immunogenic. Tumor cells were capable of delivering antigen-specific signals to T cells clone, but could not deliver costimulatory signals, e.g., a B7/CD28 interaction[25-27], necessary for full T cell activation, could lead to the evasion of immune surveillance by malignant cells[28]. Moreover, evidences have demonstrated that TILs can down-regulate the expression of CD28 molecules, but still could retain a limited immune protection in local tumor microenviroment[27]. Therefore, other molecules may involve in sustaining T cells activation and amplifying cytotoxic T lymphocytes response.
In this study, we found that 4-1BB mRNA transcripts were not detectable in normal liver, but were detected in all 19 liver tissues adjacent to tumor edge (< 1.0 cm). In some tumor and liver tissues located within 1 to 5 cm away from tumor edge, low expression of 4-1BB mRNA was also detected. Since 4-1BB molecules were mainly expressed on activated T lymphocytes[18,19], 4-1BB transcripts might derive from TILs. To identify this hypothesis, direct immunofluorescence staining of 4-1BB and CD4 or CD8 on TILs was examined by confocal microscope. Co-localization of 4-1BB+/CD4+ or CD8+ fluorescence located on TILs was visualized by confocal microscopy in tumor and liver tissue within 1 to 5 cm away from tumor edge, as well as tumor adjacent tissues within 1 cm. Previous researches reported that interaction of 4-1BB with 4-1BB ligand played an important role in sustaining T cells activation, amplifying cytotoxic T lymphocyte (CTL) response, as well as inducing IL-2 production in the complete absence of a signal through CD28 molecule[22,23]. A recent report indicated that under the condition of repeated Ag-stimulation, down-regulated expression of CD28 molecule on activated T cells could lead to activation-induced cell death (AICD)[29], while very few 4-1BB molecules might supply sufficient costimulatory signals to sustain T cells activation, and inhibit AICD[30,31]. In fact, accumulative evidence has confirmed that 4-1BB/4-1BB ligand pathway plays a role in transplant immunity[32-34] and autoimmune disease[35-37]. Therefore, the present study may provide an important clue that 4-1BB molecules are also involved in the process of infiltrating CD4+ and CD8+ T cells activation, at least partly, and that modulating 4-1BB/4-1BB ligand pathway might augment CTL response against tumor[24,38-40].
However, even if these infiltrating lymphocytes are functionally activated via 4-1BB signals or the others, and are truly specific for tumor cells, why they could not inhibit the tumour growth This phenomenon was inexplicable. Cytotoxic T cells propagated from biopsies showed a specific killing of the tumor cells in vitro, confirming that the complex microenvironment inside the tumor tissue was not able to provide the optimal condition for TILs to fully exert their functions in inhibiting tumor growth, or even to induce apoptosis of TILs through Fas/Fas ligand system[41,42].
To explore if the TILs were the same clones as T lymphocytes from peripheral blood, we analyzed the phenotypes of peripheral blood lymphocytes, and examined 4-1BB molecules and its mRNA transcripts by flow cytometry and RT-PCR respectively. To our surprise, we failed to detect 4-1BB mRNA in peripheral blood lymphocytes and 4-1BB molecules on peripheral CD4+ or CD8+ T cells from HCC patients. But our results confirmed that a significantly lower percentage of CD3+ T cells was found in HCC group as compared to healthy control group, which was coincided with other research data[43]. Since the phenotype and function of lymphocytes collected from the peripheral blood were not the same as those of lymphocytes from tumor draining regional lymph nodes and tumor tissues, we found that 4-1BB molecules were existed in infiltrating CD4+ and CD8+ T cells but not in peripheral blood lymphocytes. Therefore, we may conclude that TILs and peripheral blood lymphocytes are not the same clones[43-45].
In summary, we examined 4-1BB molecules expression in infiltrating T cells in HCC specimens, indicating that tumor infiltrating T cells can be activated via other costimulatory signals, e.g., 4-1BB, and exert a limited antitumor protection in local microenvironment. The present study also implicates that modulating 4-1BB/4-1BBL costimulatory pathway may be an effective immunotherapy strategy to augment the host response.
Edited by Zhang JZ and Wang XL
| 1. | Tang ZY, Yu YQ, Zhou XD, Ma ZC, Wu ZQ. Progress and prospects in hepatocellular carcinoma surgery. Ann Chir. 1998;52:558-563. [PubMed] |
| 2. | Zhao WH, Ma ZM, Zhou XR, Feng YZ, Fang BS. Prediction of recurrence and prognosis in patients with hepatocellular carcinoma after resection by use of CLIP score. World J Gastroenterol. 2002;8:237-242. [PubMed] |
| 3. | Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193-199. [PubMed] |
| 4. | Kanematsu T, Furui J, Yanaga K, Okudaira S, Shimada M, Shirabe K. A 16-year experience in performing hepatic resection in 303 patients with hepatocellular carcinoma: 1985-2000. Surgery. 2002;131:S153-S158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Chiappa A, Zbar AP, Audisio RA, Leone BE, Biella F, Staudacher C. Factors affecting survival and long-term outcome in the cirrhotic patient undergoing hepatic resection for hepatocellular carcinoma. Eur J Surg Oncol. 2000;26:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ouchi K, Sugawara T, Fujiya T, Kamiyama Y, Kakugawa Y, Mikuni J, Yamanami H, Nakagawa K. Prediction of recurrence and extratumor spread of hepatocellular carcinoma following resection. J Surg Oncol. 2000;75:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Philosophe B, Greig PD, Hemming AW, Cattral MS, Wanless I, Rasul I, Baxter N, Taylor BR, Langer B. Surgical management of hepatocellular carcinoma: resection or transplantation. J Gastrointest Surg. 1998;2:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Margarit C, Charco R, Hidalgo E, Allende H, Castells L, Bilbao I. Liver transplantation for malignant diseases: selection and pattern of recurrence. World J Surg. 2002;26:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Hemming AW, Cattral MS, Reed AI, Van Der Werf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther. 2002;5:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O'Day SJ, Boasberg PD, Stern SL, Ye X, Morton DL. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20:4549-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Dréno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarrière N. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Labarrière N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dréno B, Jotereau F. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother. 2002;51:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1040] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 16. | Stadler WM, Kuzel T, Dumas M, Vogelzang NJ. Multicenter phase II trial of interleukin-2, interferon-alpha, and 13-cis-retinoic acid in patients with metastatic renal-cell carcinoma. J Clin Oncol. 1998;16:1820-1825. [PubMed] |
| 17. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA. 1989;86:1963-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 292] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150:771-781. [PubMed] |
| 20. | Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 279] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Pollok KE, Kim YJ, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-μ-primed splenic B cells. Eur J Immunol. 1994;24:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551-559. [PubMed] |
| 23. | Chu NR, DeBenedette MA, Stiernholm BJ, Barber BH, Watts TH. Role of IL-12 and 4-1BB ligand in cytokine production by CD28+ and CD28_ T cells. J Immunol. 1997;158:3081-3089. [PubMed] |
| 24. | Martinet O, Ermekova V, Qiao JQ, Sauter B, Mandeli J, Chen L, Chen SH. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long- term remission of liver metastases in a mouse model. J Natl Cancer Inst. 2000;92:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Tatsumi T, Takehara T, Katayama K, Mochizuki K, Yamamoto M, Kanto T, Sasaki Y, Kasahara A, Hayashi N. Expression of costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on human hepatocellular carcinoma. Hepatology. 1997;25:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Qiu YR, Yang CL, Chen LB, Wang Q. [Analysis of CD8(+) and CD8(+)CD28(-) cell subsets in patients with hepatocellular carcinoma]. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:72-73. [PubMed] |
| 27. | Tang KF, Chan SH, Loh KS, Chong SM, Wang D, Yeoh KH, Hu H. Increased production of interferon-gamma by tumour infiltrating T lymphocytes in nasopharyngeal carcinoma: indicative of an activated status. Cancer Lett. 1999;140:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Chavan SS, Chiplunkar SV. Immunophenotypes and cytotoxic functions of lymphocytes in patients with hepatocellular carcinoma. Tumori. 1997;83:762-767. [PubMed] |
| 29. | Kim YJ, Kim SH, Mantel P, Kwon BS. Human 4-1BB regulates CD28 co-stimulation to promote Th1 cell responses. Eur J Immunol. 1998;28:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | DeBenedette MA, Chu NR, Pollok KE, Hurtado J, Wade WF, Kwon BS, Watts TH. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J Exp Med. 1995;181:985-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600-2609. [PubMed] |
| 32. | Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166:3174-3183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, Peschon JJ, Watts TH. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol. 1999;163:4833-4841. [PubMed] |
| 34. | Tan JT, Ha J, Cho HR, Tucker-Burden C, Hendrix RC, Mittler RS, Pearson TC, Larsen CP. Analysis of expression and function of the costimulatory molecule 4-1BB in alloimmune responses. Transplantation. 2000;70:175-183. [PubMed] |
| 35. | Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Michel J, Langstein J, Hofstädter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol. 1998;28:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Sharief MK. Heightened intrathecal release of soluble CD137 in patients with multiple sclerosis. Eur J Neurol. 2002;9:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Giuntoli RL, Lu J, Kobayashi H, Kennedy R, Celis E. Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin Cancer Res. 2002;8:922-931. [PubMed] |
| 39. | Ye Z, Hellström I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellström KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Martinet O, Divino CM, Zang Y, Gan Y, Mandeli J, Thung S, Pan PY, Chen SH. T cell activation with systemic agonistic antibody versus local 4-1BB ligand gene delivery combined with interleukin-12 eradicate liver metastases of breast cancer. Gene Ther. 2002;9:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Bennett MW, O'Connell J, O'Sullivan GC, Brady C, Roche D, Collins JK, Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669-5675. [PubMed] |
| 42. | Bodey B, Bodey B, Siegel SE, Kaiser HE. Immunocytochemical detection of leukocyte-associated and apoptosis-related antigen expression in childhood brain tumors. Crit Rev Oncol Hematol. 2001;39:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Santin AD, Ravaggi A, Bellone S, Pecorelli S, Cannon M, Parham GP, Hermonat PL. Tumor-infiltrating lymphocytes contain higher numbers of type 1 cytokine expressors and DR+ T cells compared with lymphocytes from tumor draining lymph nodes and peripheral blood in patients with cancer of the uterine cervix. Gynecol Oncol. 2001;81:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Echchakir H, Dorothée G, Vergnon I, Menez J, Chouaib S, Mami-Chouaib F. Cytotoxic T lymphocytes directed against a tumor-specific mutated antigen display similar HLA tetramer binding but distinct functional avidity and tissue distribution. Proc Natl Acad Sci USA. 2002;99:9358-9363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman JJ, Smith CV, Pecorelli S, Radominska-Pandya A, Cannon MJ, Parham GP. Phenotypic and functional analysis of tumor-infiltrating lympho-cytes compared with tumor-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with ad-vanced ovarian cancer. Gynecol Obstet Invest. 2001;51:254-261. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |