Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2874
Revised: December 15, 2003
Accepted: December 22, 2003
Published online: October 1, 2004
AIM: To explore a safe and efficient strategy of tumor therapy using anti-angiogenetic agents.
METHODS: Endostatin gene with a signal sequence of human IgG γ chain was amplified by PCR and cloned into pVAX1 plasmid which was the only vector authorized by FDA in clinical trial to construct a recombinant plasmid named as pVAX-sEN. The recombinant plasmid was detected with Eco I/Kpn I and DNA sequencing. BALB/c mice bearing hepatocarcinoma cell line H22 were treated with naked pVAX-sEN or liposome-DNA complex in which the dose of DNA and the ratio of DNA and liposome were different from each other. To compare the efficiency of gene transfection, expression of endostatin at the treated tumor site was assayed with ELISA. To investigate the effect of pVAX1-sEN on hepatocellular carcinoma, pVAX-sEN either naked or in liposome-DNA complex was injected into BALB/c mice bearing H22, then the diameter of tumors was measured, microvessel density was detected by immunohistochemistry, endostatin expression in vivo was assayed at different time points.
RESULTS: DNA sequencing showed the endostatin gene with the signal peptide was correctly cloned. In situ gene expression assay indicated that both the ratio of DNA and liposome and the dose of DNA could affect the gene transfection efficiency. Interestingly, naked pVAX-sEN had a similar in situ endostatin expression to pVAX-sEN with liposome. Animal experiments showed that pVAX-sEN together with pVAX-sEN-liposome complex could efficiently suppress the growth of mouse hepatoma cells.
CONCLUSION: Naked endostatin plasmid intratumoral injection can get a similar gene transfection efficiency to liposome-DNA complex when used in situ.
-
Citation: Ma CH, Zhang Y, Wang XY, Gao LF, Liu H, Guo C, Liu SX, Cao YL, Zhang LN, Sun WS. Human endostatin gene transfer, either naked or with liposome, has the same inhibitory effect on growth of mouse liver tumor cells
in vivo . World J Gastroenterol 2004; 10(19): 2874-2877 - URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2874
Tumor neovascularization is necessary to the growth and metastasis of tumors[1]. Anti-angiogenesis is an effective method to treat cancer[2,3]. Endostatin, an endogenous angiogenesis inhibitor, was found in 1997[4].It can specifically suppress endothelial cell proliferation. Animal experiments have demonstrated that endostatin can strikingly inhibit the growth of many kinds of tumor cells without drug resistance and toxicity. Endostatin is a promising agent to treat cancer[5,6]. However, to produce a large quantity of biologically active proteins has been proven difficult, the treatment requires repeated administration and high doses of recombinant protein, which limits endostatin’s clinical application. Gene therapy can overcome the above disvantages and become a potential method for cancer therapy. In this article, we constructed the recombinant eukaryotic plasmid using pVAX1 which is the only vector authorized by FDA in clinical trial. It can express secretive endostatin in vivo. Efficient gene transfer and expression are the key to gene therapy. In order to explore an efficient and easy gene transfer method, we also compared the inhibitory effect of liposome and naked DNA transfection on mouse hepatoma cells in vivo.
Eukaryotic expression vector pVAX1 authorized by FDA in clinical trial was purchased from Invitrogen(USA). Cloning vector pGEM-T-EN containing human endostatin genes was constructed and preserved in our laboratory[7].
Mouse hepatoma cell line H22 was purchased from Shandong Medical Science Institute and grown in BALB/c abdominal cavity.
BALB/c female mice, 5-6 wk of age, were provided by Experimental Animal Center of Shandong University. A total of 1 × 106 H22 cells were transplanted subcutaneously into mice. Treatment began 1 wk later, when tumor volume ranged between 0.4 to 0.5 cm3.
According to the sequences in GeneBank, primers were designed to amplify endostatin genes. In order to get a secretary protein, signal sequence of human IgG γ chain was added to the 5’ end of forward primer, and sequences recognized by Kpn I and Eco I were respectively added to the forward primer and reverse primer: forward(P1): 5’ TTAGGTACCATGGAAGCCCCAGCTCAG CTTCTCTTCCTCCTGCTACTCTGGCTCCCAGATACCACC GGACACAGCCACCGC 3’ reverse(P2): 5’ CGGCGAATTCC TTGGAGG CAGTC 3’. All of these oligonucleotide primers were synthesized by Shanghai BioAsia Bio-technology Co.Ltd.
The endostatin gene containing signal sequence of human IgG γ chain was amplified by PCR taking pGEM-T-EN as template and P1 and P2 as primers. PCR reaction was performed in 25 µL volume containing: 1 µL of plasmid DNA, 2.5 µL of 10 × buffer, 0.5 mmoL Mg2+, 5 nmol/L dNTP, 3 units of Taq polymerase,10 nmol/L primers (forward and reverse). Reaction conditions were at 95 °C for 5 min, then 30 cycles each at 95 °C for 1 min, at 62 °C for 1 min, at 72 °C for 3 min, followed by a final extension at 72 °C for 5 min. PCR products were purified by UNIQUE-10 Kit (Shanghai Shenggong Biological Co.Ltd) after 20 g/L agarose gel electrophoresis.
PCR products and pVAX1 were digested by EcoR I and Kpn I (TaKaRa Biotechnology, Dalian) respectively, then purifed and ligased with T4DNA ligase (TaKaRa Biotechnology, Dalian) at room temperature overnight. E.coli, DH5α, competent cells were transfected using CaCl2. Recombinant clones were detected by EcoR I and Kpn I and then sequenced by Shanghai BioAsia Bio technology Co.Ltd.
Five-to-six week old female BALB/c mice were injected subcutaneously with 1 × 106 logarithmically growing H22 cells to axillary. One week later, when tumor volume was about 0.4 to 0.5 cm3, naked plasmid or liposome-DNA complex (LipofectamineTM2000 from Invitrogen) was administered intratumorly. The ratio of liposome and recombinant plasmid in liposome-DNA complex was 1:1, 1:3, 1:8 respectively. Animals were sacrificed 3 d after injection and about 0.5 cm3 of tumor was separated and cut into pieces ,then cultured in 0.5 mL MEM medium with 100 mL/L of FCS at 37 °C, 50 mL/L CO2 atmosphere for 24 h. Supernatants were collected and centrifuged at 1000 r/min for 5 min, then stored at -20 °C for gene expression assay.
One week after BALB/c mice were injected subcutaneously with 1 × 106 logarithmically growing H22 cells to axillary, naked recombinant plasmid (20 µg) or the liposome-DNA complex containing 20 µg recombinant plasmid was administered intratumorly once a week for two weeks. The ratio of liposome and recombinant plasmid in liposome-DNA complex was 1:1. pVAX1 and NS were respectively injected into animals as control. The length (a) and width (b) of tumor were measured twice a week. The tumor volumes were then calculated using the formula: Volume = a × b2× 0.52.
The experiment was done in triplicate with 5 animals in each group.
In order to determine the association of anti-tumor effects and the expression level of endostatin, peripheral blood was prepared from tail at different time point and serum was used to detect the expression of endostatin with ELISA.
Expression of endostatin was detected with human endostatinTM protein accucyteR EIA kit (Oncogene) following the manufacturer’s instructions.
Mice were killed 3 d after the fourth administration. Tumors were excised, fixed in formalin and prepared for paraffin-embedded sections. Factor VIII-related antigen was detected with S-P immunohistochemistory kit (Beijing Zhongshan Biocompany) according to the manufacturer’s instructions. Tumor microvessel density (MVD) was evaluated as described by Weidner[8].
All data were expressed as mean ± SD. Student’s t -test was used for statistical analysis. A P value < 0.05 was considered statistically significant.
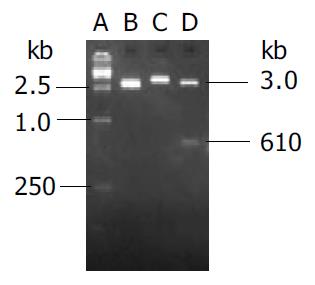
Endostatin gene with signal sequence of human IgG γ chain was amplified by PCR and cloned into eukaryotic vector pVAX1 named as pVAX-sEN. Two percent agarose gel electrophoresis demonstrated two binds about 3 kb and 610 bp in size as expected after the recombinant plasmid was digested by Kpn I and EcoR I (Figure 1). DNA sequencing analysis indicated both the sequence of endostatin and signal peptide were in accordance with those in the GenBank.
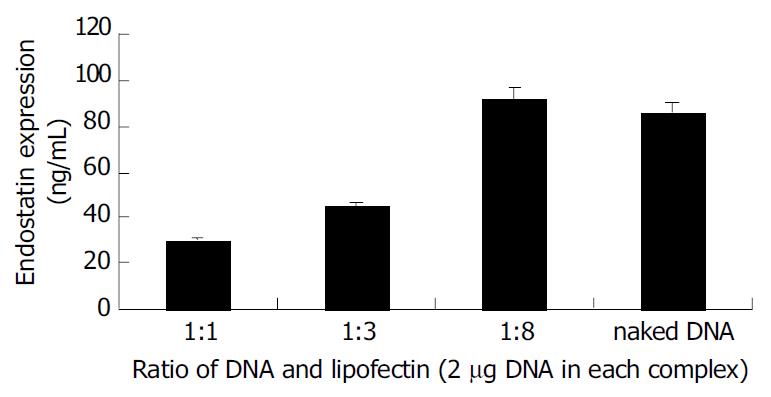
To compare the efficiency of gene transfection, expression of endostatin transgenic protein at the tumor site of the treated group was assayed with ELISA. Endostatins were detected in all samples treated with naked or liposome-DNA complex but not in the control groups (Figure 2). Endostatin expression was about 30 ng/mL when the ratio of liposome and DNA was 1:1. With the addition of the liposome into complex the expression of endostatin was increased gradually. When the ratio reached 1:8, the endostatin was 92 ng/mL. Interestingly, naked DNA administration could get a pretty high gene expression which was about 86 ng/mL.
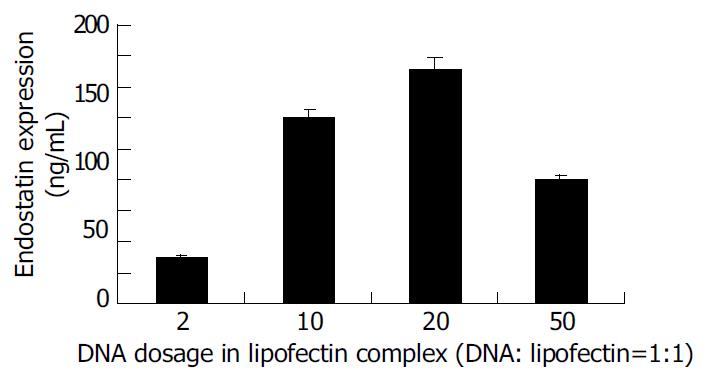
To compare gene transfection using liposome-DNA complex containing different doses of DNA in vivo, liposome-DNA complex was prepared with the ratio of liposome and DNA 1:1 which contained different doses of DNA. Twenty-four hours after transfection endostatins were detected by ELISA (Figure 3). The result indicated that when DNA was less than 20 µg , with the elevation of DNA doses, the expression of endostatin increased. When the DNA dose reached 20 µg, endostatin reached 152 ng/mL, the peak expression. On the contrary, when the DNA reached 50 µg, the expression of endostatin dropped to 80 ng/mL.
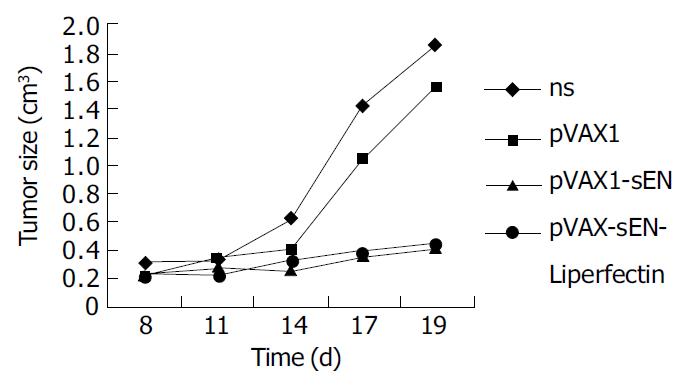
Changes of tumor volumes indicated pVAX-sEN-liposome complex could obviously inhibit the growth of mouse hepatoma cells when administered intratumorly (Figure 4). The tumor volume was only 0.451 ± 0.26 cm 3, obviously less than that in NS treated group (1.86 ± 0.62 cm 3) and pVAX1 treated group (1.56 ± 0.37 cm 3) (P < 0.05). The inhibition rate was about 73%. Interestingly, pVAX-sEN alone could produce an inhibitory effect. The tumor size in pVAX-sEN treated group was 0.4 ± 0.25 cm 3, and the inhibitory rate was about 80%, which was not significant from pVAX-sEN-liposome complex treated group (P > 0.05).
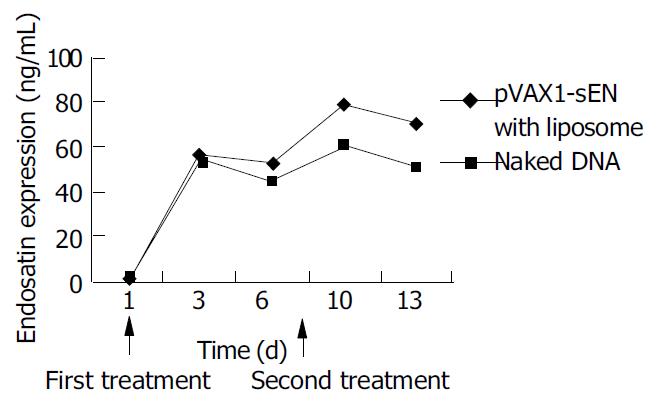
ELISA showed that the expression of endostatin in peripheral blood varied with the administration of recombinant plasmid. Three days after the first treatment of recombinant plasmid, both naked and in combination with liposome, the expression of endostatin could be detected (56.8 ± 3.8 ng/mL and 54 ± 5.8 ng/mL, respectively. P > 0.05) and then the expression decreased 2 d later. After the second administration the expression raised to 79.8 ± 6.1 ng/mL and 60.43 ± 8.3 ng/mL and it could be lasted for another 3 d. The expression level in naked plasmid treated mice was lower than that in DNA-liposome complex treated mice (50.1 ± 6.3 ng/mL vs 70.4 ± 6.4 ng/mL, P < 0.05) (Figure 5).
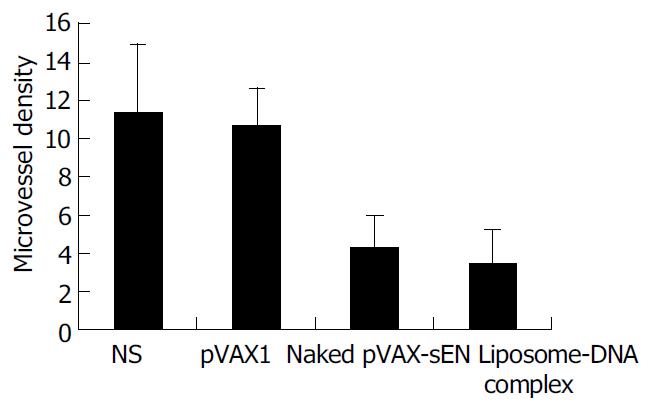
Immunohistochemical analysis showed a potent inhibition of angiogenesis in tumors treated by pVAX-sEN. The MVD was counted and results are shown in Figure 6. Expression of endostatin mediated by naked pVAX-sEN or liposome-DNA complex injection caused a significant reduction in MVD compared with the NS control group or the pVAX1 treated group (P < 0.05). However, the difference in MVD between naked DNA and liposome-DNA complex injection groups was not statistically significant.
Growth and metastasis of tumor depend on the neuvascularization. Anti-angiogenesis can effectively suppress tumor growth. Endostatin was found to specifically inhibit endothelial cell proliferation but not actively proliferating cells such as carcinoma cells, intestinal cells, epithelial cells, bone marrow cells[4]. So the application of endostatin did not cause bone marrow ablation, stomach and intestinal reaction, and other side effects[5]. Moreover, endostatin could be used for a long time without drug resistance since its target cells, endothelial cells have a relatively stable gene structure and low mutation rate[5,6]. Consequently endostatin is an attractive new strategy in cancer therapy.
Recombinant endostatin protein has been used in clinical trials at present[9]. However poor solubility of recombinant endostatin protein and its high effective dose have limited its wide spread application[4]. Gene therapy by which endostatin can express in vivo is an efficient method for anti-angiogenesis cancer therapy[10-12].
The key point of gene therapy is to establish an effective gene delivering system. Vectors used to deliver genes nowadays mainly include viral and non-viral vectors. Because of limitations of viral vectors such as potent danger, immune response and limitations of the gene length[13-15], more and more researchers have payed their attention to non-viral vectors. In this paper, a highly safe vector pVAX1 authorized by FDA in clinical trial was selected to construct a recombinant vector expressing secretive endostatin which was named as pVAX-sEN. DNA sequencing indicated endostatin gene together with a signal peptide was correctly cloned. Animal experiments showed pVAX-sEN could efficiently suppress the growth of mouse hepatoma cells. This study provides an experimental basis for further study on safe and efficient gene therapy with endostatin.
Non-viral gene can be transferred by physical and chemical methods. The cationic liposome-mediated gene transfer system is popular because of its low immunity, high safety, easy preparation, no limitation to gene sequence and potential to be used repeatedly[16,17]. Gene transfer efficiency of liposome can be affected by many factors. Studies indicated that a suitable quantity ratio of DNA and liposome was one of the essential factors[18]. Naked DNA injection is another simple safe gene transfer method. Many researchers have verified that naked DNA administration could induce a high gene expression level[19,20]. In 2003 VEGF administered into cardiac muscle using naked DNA was applied to clinical trial[21]. In our study, cationic liposome was used to transfer endostatin DNA into mouse hepatoma cells in vivo. It showed that when the quantity ratio of liposome and DNA was 1:1, the expression of endostatin was pretty high. When the quantity ratio was 1:8, endostatin reached the highest expression (92 ng/mL). With the elevation of DNA dose, the expression of endostatin increased. When the DNA dose reached 20 µg, the expression of endostatin amounted to 152 ng/mL. However, if DNA dose reached 50 µg, the expression of endostatin dropped to 80 ng/mL. The possible reason was that the high dose of liposome in the complex might be deposited[18]. So the transfection efficiency was lower. Results also indicated that naked DNA could also get a pretty high gene expression (86 ng/mL). Our in vivo inhibition assay even showed that naked DNA could get an inhibition as efficient as DNA-liposome complex.In 2002, Shi et al[22] compared the transfection rate of naked DNA with liposome-DNA complex and found the expression of IL-12 was equal. Their result is in accordance with ours. However, in vivo gene expression assay indicated that the peripheral expression of endostatin in naked DNA treated mice decreased more quickly than that in DNA-liposome complex treated mice.
In conclusion, we successfully constructed the recombinant vector secretively expressing endostatin using highly safe vector pVAX1 named pVAX-sEN. Animal experiments showed it could efficiently suppress the growth of hepatoma cells. It is a promising method in cancer therapy. At the same time we compared the transfection efficiency of naked pVAX-sEN DNA with liposome-DNA complex. Results indicated naked DNA could produce both a high endostatin gene expression and a good tumor inhibition effect when used in situ, strongly suggesting that naked DNA administration is a simple, safe and efficient gene transfer method.
Edited by Zhu LH and Wang XL Proofread by Xu FM
| 1. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 3194] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 2. | Marx J. Angiogenesis. A boost for tumor starvation. Science. 2003;301:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Brem S, Brem H, Folkman J, Finkelstein D, Patz A. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res. 1976;36:2807-2812. [PubMed] |
| 4. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3111] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 5. | Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1201] [Cited by in RCA: 1130] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 6. | Kerbel RS. A cancer therapy resistant to resistance. Nature. 1997;390:335-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 211] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Ma CH, Sun WS, Zhang LN, Wang XY, Li X. Expression of human endostatin in picha yeast and its inhibition effect on tumor. J Shandong Med University. 2001;39:334-336. |
| 8. | Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 565] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Eder JP, Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, Shulman LN, Proper J, Kirvan M, Rattner B. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol. 2002;20:3772-3784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Wang X, Liu F, Li X, Li J, Xu G. Anti-tumor effect of human endostatin mediated by retroviral gene transfer in nude mice. Chin Med J (Engl). 2002;115:1664-1669. [PubMed] |
| 11. | Nakashima Y, Yano M, Kobayashi Y, Moriyama S, Sasaki H, Toyama T, Yamashita H, Fukai I, Iwase H, Yamakawa Y. Endostatin gene therapy on murine lung metastases model utilizing cationic vector-mediated intravenous gene delivery. Gene Ther. 2003;10:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Li X, Fu GF, Fan YR, Shi CF, Liu XJ, Xu GX, Wang JJ. Potent inhibition of angiogenesis and liver tumor growth by administration of an aerosol containing a transferrin-liposome-endostatin complex. World J Gastroenterol. 2003;9:262-266. [PubMed] |
| 13. | Bramson JL, Hitt M, Gauldie J, Graham FL. Pre-existing immunity to adenovirus does not prevent tumor regression following intratumoral administration of a vector expressing IL-12 but inhibits virus dissemination. Gene Ther. 1997;4:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 368] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Rochlitz CF. Gene therapy of cancer. Drugs Today (Barc). 2000;36:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ota T, Maeda M, Tatsuka M. Cationic liposomes with plasmid DNA influence cancer metastatic capability. Anticancer Res. 2002;22:4049-4052. [PubMed] |
| 17. | Kaiser S, Toborek M. Liposome-mediated high-efficiency transfection of human endothelial cells. J Vasc Res. 2001;38:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Clark PR, Stopeck AT, Ferrari M, Parker SE, Hersh EM. Studies of direct intratumoral gene transfer using cationic lipid-complexed plasmid DNA. Cancer Gene Ther. 2000;7:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Kim JM, Jeong JG, Ho SH, Hahn W, Park EJ, Kim S, Yu SS, Lee YW, Kim S. Protection against collagen-induced arthritis by intramuscular gene therapy with an expression plasmid for the interleukin-1 receptor antagonist. Gene Ther. 2003;10:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Imboden M, Shi F, Pugh TD, Freud AG, Thom NJ, Hank JA, Hao Z, Staelin ST, Sondel PM, Mahvi DM. Safety of interleukin-12 gene therapy against cancer: a murine biodistribution and toxicity study. Hum Gene Ther. 2003;14:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ, Schaer GL, March R, Snell RJ, Henry TD. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol. 2003;92:436-439. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Shi F, Rakhmilevich AL, Heise CP, Oshikawa K, Sondel PM, Yang NS, Mahvi DM. Intratumoral injection of interleukin-12 plasmid DNA, either naked or in complex with cationic lipid, results in similar tumor regression in a murine model. Mol Cancer Ther. 2002;1:949-957. [PubMed] |