Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2870
Revised: December 9, 2003
Accepted: December 16, 2003
Published online: October 1, 2004
AIM: To explore the ability of recombinant toxin luteinizing hormone releasing hormone-Pseudomonas aeruginosa exotoxin 40 (LHRH-PE40) and LHRH binding to LHRH receptor (LHRHR) on the membrane surface of human liver cancer HEPG cells.
METHODS: LHRH was labeled by using 125I with enzymatic reaction. The affinity and receptor volume of LHRH-PE40 and LHRH binding to LHRHR on the membrane surface of human liver cancer cells were measured with radioligand receptor assay.
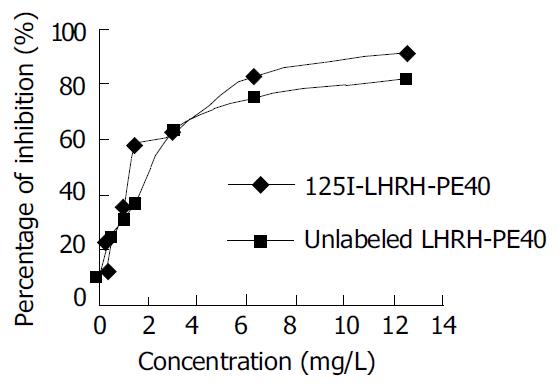
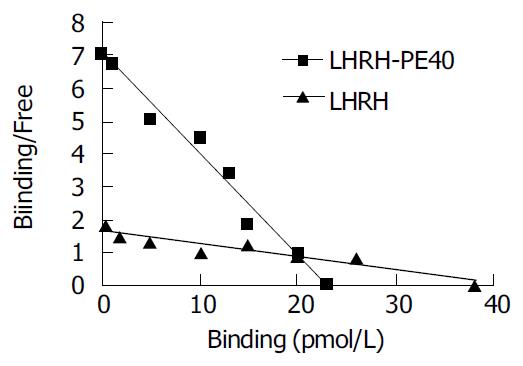
RESULTS: The specific activity of LHRH labeled with 125I was 2.7 × 104 kBq/μL, and its radiochemical purity reached to 99.2%-99.7%. The binding of 125I to LHRH was maximal for 240 min in the warm cultivation, and this binding was stabilized. The inhibiting rates of 125I-LHRH and LHRH on the proliferation of human liver cancer HEPG cells were not significantly different. On the basis of the saturation curve of 125I-LHRH binding to the membrane LHRHR of HEPG cells, 125I-LHRH of 1 × 105 cpm was selected for radioligand receptor assay. The affinity constants (Kd) of LHRH-PE40 and LHRH binding to the membrane LHRHR of HEPG cells were 0.43 ± 0.12 nmol/L and 4.86 ± 1.47 nmol/L, respectively, and their receptor volumes were 0.37 ± 0.15 μmol/g and 0.42 ± 0.13 μmol/g, respectively. The binding of LHRH-PE40 to the membrane protein of normal liver cells was not observed.
CONCLUSION: The recombinant toxin LHRH-PE40 binding to the membrane surface of LHRHR of human liver cancer HEPG cells was very strong, while the specific binding of it to normal liver cells was not observed. The results provide an important experimental basis for the clinical application of LHRH-PE.
-
Citation: Gong SL, Zhao G, Zhao HG, Lü WT, Liu GW, Zhu P. Ability of luteinizing hormone releasing hormone-
Pseudomonas aeruginosa exotoxin 40 binding to LHRH receptor on human liver cancer cells. World J Gastroenterol 2004; 10(19): 2870-2873 - URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2870
Guide medicine is currently one of the focuses in the field of medicine and biology. We can couple some tumor specific antibodies, cytokines or hormones, etc. with toxins (such as animal and plant toxin, radiotherapy and chemotherapy medicines or bacterial toxins, etc.) through chemical method or connection by gene fusing technology, and then express fusing protein to make a guide medicine with specific target characteristics and killing cell toxicity, that is biological missile, which can selectively kill homologous antigen-associated cells or antibody-associated cells, while exerts little effect on irrespective cells[1]. So far, a series of recombined immune toxins have shown perfect effects through in vitro and animal experiment[2-4], moreover the results in some clinic investigations are also satisfactory[5,6]. DAB389IL-2 which IL-2 receptor used as a guide medicine of target has passed FAD’s warrant and come into use[7].
Luteinizing hormone-releasing hormone (LHRH) is also termed gonadotropin-releasing hormone (GnRH). It is a peptide hormone synthesized and secreted mostly from hypothalamus. Pseudomonas aeruginosa exotoxin 40 (PE40) was obtained by removing I a region of PE. A fusing protein LHRH-PE40 was recombined by the fusion of both LHRH gene and PE40 gene with genetic engineering in vitro. LHRH-PE40 is a guide medicine to treat cancer. PE40 has effect on antitumor and immune regulation. The key of LHRH-PE40 biological effect is selectively to kill carcinoma cells. This effect is gained by the distributing difference of membrane surface LHRH receptor (LHRHR) in live cancer cells and normal liver cells. Many experimental results showed that LHRHR expressed greatly in the membrane surface of some carcinoma cells, but not in that of normal liver cells[8-13]. The results provide an important basis for studying and preparing guide medicine to treat cancer[14]. Therefore, LHRHR is acted as a sign to distinguish carcinoma cells from normal cells. The abilities of LHRH-PE40 binding to the membrane surface LHRHR of liver cancer cells and normal liver cells were observed with radioligand receptor assay for providing an important experimental basis for LHRH-PE40 clinical application.
Human liver cancer HEPG cell line was bought from the Cell Center, Institute of Cell Biology, Chinese Academy of Medical Sciences (Beijing, China). Human liver tissues (3 cases) were provided by the Center of Medicolegal Examination, Criminal and Police Team, Liaoyuan City, Jilin, China.
Human liver cancer HEPG cell line was maintained in 75 cm2 culture flasks at 37 °C in a humidified 50 mL/L CO2-in-air mixture. The cells were passed weekly. After cells were harvested in exponential growth phase, they were dissociated with a non-enzymatic cell dissociation solution (Sigma, USA), and then counted by a hemacytometer. The cells were washed twice with phosphate-buffered saline (PBS) before cell membrane protein was prepared.
125I was from Amersham, UK. LHRH and thyrotropin releasing hormone (TRH) were bought from Institute of Biochemistry, Shanghai. LHRH-PE40 was synthesized by the Institute of Military Veterinary Medicine, Quartermaster University of PLA. Lactoperoxidase, glucoseoxidase and Sephadex G-25 gel were made in Sigma, USA.
The lactoperoxidase-glucoseoxidase method by Piyachaturawata et al[15] was slightly modified to label LHRH using 125I with enzymatic reaction. Half mol/L PBS of 20 µL (pH7.6), LHRH of 5 µg (dissolved in 0.1 mol/L NaAc of 5 µL), 2.3 mU lactoperoxidase (dissolved in 0.1 mol/L NaAc of 10 µL, pH5.6), 2.8 mU glucoseozidase (dissolved in 0.1 mol/L NaAc of 10 µL, pH5.6) and 5 g/L β-D (+) glucose of 5 µL in proper order were added into the test tube with 3.7 × 104 kBq fresh 125I of non-reducer, oscillated and mixed, to make 125I quickly labeling to LHRH. After the reaction for 5 min at 22-25 °C, 0.1 mol/L boric acid buffer of 50 µL was added into the test tube to stop the reaction. The reactant of LHRH labeled with 125I in test tube was separated and purified by column chromatography (the column of 1 cm%-25 cm) with Sephadex G-25 gel to removing free 125I, eluted with o.1 mol/L boric acid buffer (containing 1 g/L gelatin and 0.2 g/L sodium azide, pH9.2, flow rate: 3 mL/3 min, 3 mL/tube). Thirty-six tubes were collected. The radioactivity (counts per minute, cpm) of 125I-LHRH of 10 µL in each tube was determined. The specific activity of 125I-LHRH determined with self-displacement curve was 2.69-3.7 × 104 GBq/g LHRH.
Inhibiting rates of both 125I-LHRH and LHRH on the proliferation of human liver cancer HEPG cells were measured using enzyme-linked immunosorbent assay meter (Bio-Rad, USA) with MTT (thiazolyl blue) method[16].
The preparation of the membrane protein in human liver cancer HEPG cells: The cells were centrifuged in culture fluid, counted, washed once with PBS and centrifuged (500 r/min, 4 °C, 5 min). The centrifuged sediment was suspended in ice-cold sucrose buffer for 20 min. The suspension was again centrifuged (300 r/min, 4 °C, 5 min) after the cells in the suspension were ground with ultrasonic meter at ice bath. The centrifuged supernatant was further centrifuged (18000 g, 4 °C, 30 min). The centrifuged sediment was a membrane protein. Then the purer membrane protein of liver cancer cells was gained after the membrane protein was washed once with PBS.
Preparation of membrane protein in normal human liver cells: The liver tissue was sheared into fragments with ophthalmic scissors after the frozen tissue was thawed in sucrose buffer. The fragments were homogenized at ice bath for 1 min and centrifuged (1500 r/min, 4 °C, 10 min). The centrifuged supernatant was again centrifuged (31000 g, 4 °C, 15 min). Then the purer membrane protein of normal liver cells was gained after the membrane protein was washed once with PBS.
Polypropylene test tubes dried after soak with 10 g/L bovine serum albumin (BSA) were used as the reactive tubes. The tri-tube was used as a determined value. LHRH-PE40 or LHRH of different concentrations and 125I-LHRH (1 × 105 cpm) of 100 µL in proper order were added into the reactive tube in ice bath, respectively, and mixed. Then, 100 µL suspension of the cell membrane (containing 180-220 µg protein) was added into the reactive tube. The reactive tubes were cultivated at 4 °C for 4 h after oscillation. Excessive LHRH-PE40 or LHRH (200 µg) was added into the non-specific binding tube. The total volume in the reactive tube was 0.3 mL. The reactant was filtrated by using a microfilter with 32# glass fiber filter paper at 93.3 kPa negative pressure vacuum. The reactive tube and filter pore were washed 3 times with 0.5 mL of precolded 0.5 mol/L PBS (pH7.4): physiological saline at 1:50. The radioactivity of the sediment on the filter paper filtered at vacuum and dried for 5 min was measured with 125I automatic radio-immunity analyzer. The protein was measured with Lowry modification, and its standard curve was done with human serum albumin.
The specific activity of LHRH labeled with 125I of enzymatic reaction was 2.7 × 104 kBq/µg. Its radiochemical purity measured using paper chromatography with the developer of 5% KI and 1 mol/L HCl (1:1) reached 99.2%-99.7%. 125I-LHRH did not produce free 125I 3 and 7 d after preservation at -80 °C, and produced 5% free 125I after 10 d. The inhibiting rate of 125I-LHRH on the proliferation of cancer cells was not significantly different as compared with that of LHRH (Figure 1).
The 125I-LHRH binding to HEPG cell membrane protein was observed for 60, 90, 120, 150, 180, 240 and 300 min in warm cultivation. The binding increased with the prolongation of warm cultivation time, and reached the peak value at 240 min. If the time in the warm cultivation was prolonged further, the binding decreased.
The specific binding of HEPG cell membrane protein to 125I-LHRH increased with the protein concentration. The increase of the binding was not obvious as protein concentration exceeded 250 µg per 0.3 mL, while the non-specific binding increased obviously. According to the experimental results, the protein concentration of 180-220 µg per 0.3 mL was used as radioligand receptor assay.
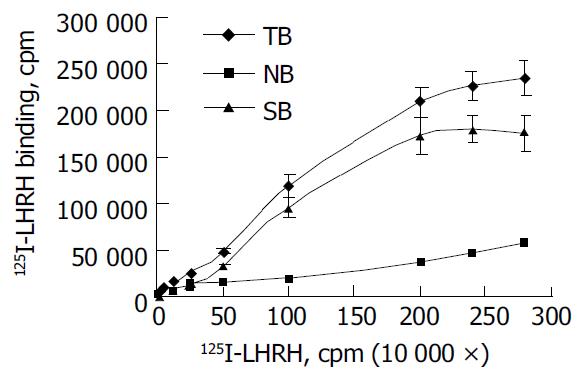
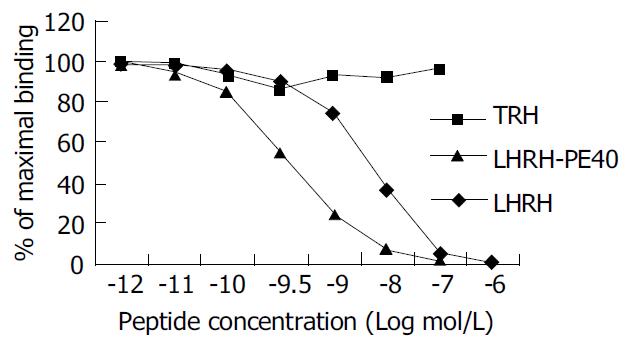
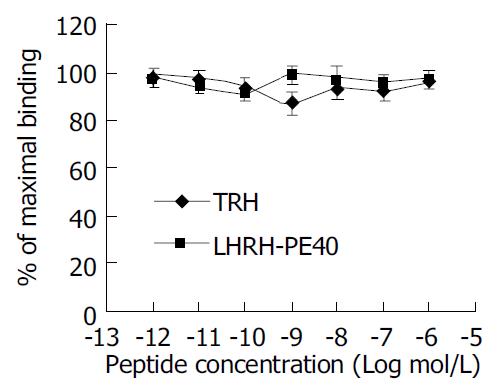
The saturation curve of 125I-LHRH binding to LHRHR of HEPG cells was firstly done. 125I-LHRH of 1 × 105 cpm in 50% linear range of the saturation curve was selected as radioligand receptor assay (Figure 2). Figure 3 shows the inhibiting curve of competitive binding of non-labeled LHRH-PE40 and LHRH to HEPG cell membrane protein LHRHR with 125I-LHRH. 125I-LHRH binding to LHRHR reduced with increase of the non-labeled ligands to demonstrate a specific inhibiting curve of competitive binding. LHRH-PE40 could bind to HEPG cell surface LHRHR, while TRH could not. Figure 4 was drawn with Scatchard method to gain two straight lines. The affinity constants (Kd) of binding of LHRH-PE40 and LHRH to LHRHR of HEPG cells were 0.43 ± 0.12 µmol/g and 4.86 ± 1.47 µmol/g, respectively, and their receptor volumes were 0.37 ± 0.15 µmol/g and 0.42 ± 0.13 µmol/g, respectively.
The binding curve of LHRH-PE40 to the membrane protein of normal liver cells was not significantly different from that of negative control TRH (Figure 5). The labeled ligand 125I-LHRH binding to the membrane protein of normal liver cells did not change with increase of the non-labeled ligand LHRH-PE40. The result suggested that LHRH-PE40 binding to trace LHRHR in the membrane of normal human liver cells could not be examined.
The specific activity of LHRH labeled with 125I of enzymatic reaction was 2.7 × 104 kBq/µL, and its radiochemical purity reached 99.2%-99.7%. The 125I binding to LHRH was maximal at 240 min in the warm cultivation. This binding was stabilized, i.e.125I-LHRH did not produce free 125I 3 and 7 d after preservation at -80 °C, and produced 5% free 125I after 10 d. The inhibiting rates of 125I-LHRH and LHRH on the proliferation of human liver cancer HEPG cells measured with MTT (thiazolyl blue) method were not significantly different. The results suggest that LHRH satisfactorily labeled with 125I of enzymatic reaction may be used as radioligand receptor assay.
LHRH-PE40 is a guide medicine to treat cancer. It is composed of the guide fraction of LHRH peptides and the toxicity fraction of PE40 protein. The active principle of LHRH-PE40 is that its toxicity fraction PE40 enters in cells through transmembrane transport to kill cancer cells after its guide fraction LHRH binds to LHRHR of cancer cell membrane surface. The expression of LHRH and its receptor as a part of a negative autocrine regulatory system of cell proliferation has been demonstrated in a number of human malignant tumors. Moreover, the dose-dependent antiproliferative effects of LHRH agonists on cell lines derived from these cancers have been observed by various investigators[17-19]. The effect exerts through the signal transduction in cancer cells[20,21]. So, the specific binding of guide fraction LHRH to LHRHR of cancer cell membrane surface becomes the key in the treatment of carcinoma. It has been currently demonstrated that there also are LHRH and its receptor LHRHR in some tissues besides hypothalamus and pituitary. Some literatures indicated that there was LHRHR in the membrane surface of normal liver cells, but its content was very low[8,9]. The fact brings forward a challenge against the LHRHR specificity of killing cancer cells. However, our experimental results showed that specific binding of LHRH-PE40 to the membrane surface LHRHR of human liver cancer HEPG cells occurred, while did not to normal human liver cell membrane surface where there may be trace LHRHR. Of course, the negative result of this non-specific binding may be affected by the precision limitation in the experimental method itself. For example, some methods could be used only when the Kd of sample was less than 10-8 nmol/L[22]. Both epidermal growth factor (EGF) and interleukin-2 (IL-2) used as the guide medicines are also faced with the same problem that their trace receptors exist on the surface of normal liver and kidney cells, but they have been used in clinic. Although this kind of medicines may also have a weak liver toxicity, their side effects are less as compared with chemotherapy. So, LHRH-PE40 has some value in curing liver cancer with numeric proliferation of LHRHR.
The binding of hormone to its receptor was limited by many factors[23]. Among these factors the space of hormone protein can directly affect the binding to its receptor. LHRH is a decapeptide without free amino and carboxyl. The amino acids at its 4-6 sites can form β -sheet like a barrette, which makes it binding to receptor. Furthermore, the amino acids at this hormone’s first and 4-10 sites can bind to its receptor too. LHRH of LHRH-PE40 replaces I a section of PE. A linking bridge of both His and Met amino acids added between two molecules maintains the primary structure of LHRH. But whether LHRH’s space configuration of this fusing protein changes should be further explored. The results from this experiment were that the affinity constants (Kd) of LHRH-PE40 and LHRH binding to LHRHR of HEPG cells were 0.43 ± 0.12 nmol/g and 4.86 ± 1.47 nmol/L, respectively, and their receptor volumes were 0.37 ± 0.15 μmol/g and 0.42 ± 0.13 μmol/g, respectively. The affinity constant of LHRH-PE40 is nearly one-tenth of LHRH. Therefore, we can infer that LHRH-PE40 maintains the characteristics of LHRH binding to its receptor, and its binding capacity is stronger than LHRH itself. The result is identical with the report published elsewhere[6]. This enhancing binding capacity may be caused by its molecular mass being larger than LHRH, which makes itself more stable and decomposed difficultly in vitro. These results proved that LHRH-PE40 can bind to LHRHR on the surface of liver cancer cells, and then exerts its guide action.
Up till now, some problems still need to be resolved on the guide medicine for the treatment of cancer. Somebody testified that positive rate of LHRHR in adjacent tissues of cancers was even higher than that in cancer tissues[24], which should be attached importance. The articles demonstrated that the signal transduction pathways mediated by LHRHR were different[25], LHRHR of cancer cell surface could mediate cell apoptosis[26,27].
We are grateful to Dr. Zhang PY and Dr. Li JZ for their excellent technical assistance, and Dr. Wu GM and Dr. Zhang GL for their providing experimental materials.
Edited by Xu CT and Wang XL Proofread by Pan BR and Xu FM
| 1. | Panchal RG. Novel therapeutic strategies to selectively kill cancer cells. Biochem Pharmacol. 1998;55:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Reiter Y, Pastan I. Recombinant Fv immunotoxins and Fv fragments as novel agents for cancer therapy and diagnosis. Trends Biotechnol. 1998;16:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Perentesis JP, Gunther R, Waurzyniak B, Yanishevski Y, Myers DE, Ek O, Messinger Y, Shao Y, Chelstrom LM, Schneider E. In vivo biotherapy of HL-60 myeloid leukemia with a genetically engineered recombinant fusion toxin directed against the human granulocyte macrophage colony-stimulating factor receptor. Clin Cancer Res. 1997;3:2217-2227. [PubMed] |
| 4. | Saleh MN, LeMaistre CF, Kuzel TM, Foss F, Platanias LC, Schwartz G, Ratain M, Rook A, Freytes CO, Craig F. Antitumor activity of DAB389IL-2 fusion toxin in mycosis fungoides. J Am Acad Dermatol. 1998;39:63-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Kreitman RJ, Wilson WH, Robbins D, Margulies I, Stetler-Stevenson M, Waldmann TA, Pastan I. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340-3348. [PubMed] |
| 6. | Haggerty HG, Warner WA, Comereski CR, Peden WM, Mezza LE, Damle BD, Siegall CB, Davidson TJ. BR96 sFv-PE40 immunotoxin: nonclinical safety assessment. Toxicol Pathol. 1999;27:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Duvic M, Kuzel TM, Olsen EA, Martin AG, Foss FM, Kim YH, Heald PW, Bacha P, Nichols J, Liepa A. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK). Clin Lymphoma. 2002;2:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Zhao G, Gong SL, Yue Y, Zhu P. Distribution of human LHRH receptors in normal and carcinomatous tissues outside pituitary. Jilin Daxue Xuebao. 2002;28:445-447. |
| 9. | Zhao G, Gong SL, Zhu P. Binding ability of LHRH-PE40 to LHRH receptors on the surface of cancer cell line. Jilin Daxue Xuebao. 2003;29:5-8. |
| 10. | Dharap SS, Qiu B, Williams GC, Sinko P, Stein S, Minko T. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J Control Release. 2003;91:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Gründker C, Günthert AR, Millar RP, Emons G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J Clin Endocrinol Metab. 2002;87:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Volker P, Grundker C, Schmidt O, Schulz KD, Emons G. Ex-pression of receptors for luteinizing hormone-releasing hor-mone in human ovarian and endometrial cancers: frequency, autoregulation, and correlation with direct antiproliferative activity of luteinizing hormone-releasing hormone analogues. Am J Obstet Gynecol. 2002;186:171–179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000;163:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Plonowski A, Schally AV, Nagy A, Groot K, Krupa M, Navone NM, Logothetis C. Inhibition of in vivo proliferation of MDA-PCa-2b human prostate cancer by a targeted cytotoxic analog of luteinizing hormone-releasing hormone AN-207. Cancer Lett. 2002;176:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Piyachaturawata P, Pedroza E, Huang WY, Arimura A, Schally AV. Studies on the iodination of LH-RH and the biological and immunological activities of the products. Life Sci. 1980;26:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Xu N, Shi AP, Wang YD, Wang CX, Guo WH. Effect of telomerase oligonucleotides on the inhibition of telomerase ac-tivity and cell growth in bladder. Jilin Daxue Xuebao. 2003;29:630-633. |
| 17. | Günthert AR, Gründker C, Hollmann K, Emons G. Luteinizing hormone-releasing hormone induces JunD-DNA binding and extends cell cycle in human ovarian cancer cells. Biochem Biophys Res Commun. 2002;294:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Gründker C, Günthert AR, Westphalen S, Emons G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur J Endocrinol. 2002;146:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Shacham S, Cheifetz MN, Lewy H, Ashkenazi IE, Becker OM, Seger R, Naor Z. Mechanism of GnRH receptor signaling: from the membrane to the nucleus. Ann Endocrinol (Paris). 1999;60:79-88. [PubMed] |
| 20. | Gründker C, Schlotawa L, Viereck V, Emons G. Protein kinase C-independent stimulation of activator protein-1 and c-Jun N-terminal kinase activity in human endometrial cancer cells by the LHRH agonist triptorelin. Eur J Endocrinol. 2001;145:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Gründker C, Völker P, Schulz KD, Emons G. Luteinizing hormone-releasing hormone agonist triptorelin and antagonist cetrorelix inhibit EGF-induced c-fos expression in human gynecological cancers. Gynecol Oncol. 2000;78:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Lü BZ, Tian Y. Receptorology outline. 1st Ed. Beijing Sciences Press. 1991;27-32. |
| 23. | Luo HB, Zhu P, Li LJ. Bacterial toxin and clinic. 1st Ed. Beijing People Health Press. 1999;91-99. |
| 24. | Zhang JS, Huang GS, Huang WQ, Zhang YQ. Immunohiso-chemical localization of gonadotropin releasing hormone and its receptor in hepatocellular carcinoma tissues. Disi Junyi Daxue Xuebao. 1998;19:235-236. |
| 25. | Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology. 1999;140:5250-5256. [PubMed] [DOI] [Full Text] |
| 26. | Imai A, Tamaya T. GnRH receptor and apoptotic signaling. Vitam Horm. 2000;59:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Gründker C, Schulz K, Günthert AR, Emons G. Luteinizing hormone-releasing hormone induces nuclear factor kappaB-activation and inhibits apoptosis in ovarian cancer cells. J Clin Endocrinol Metab. 2000;85:3815-3820. [PubMed] |